AZ's Seroquel Battle with FDA: A Mark of Desperation?
Is it a mark of desperation? UK-based pharma company AstraZeneca has resorted to suing the US Food and Drug Administration in a bid to stop it from approving generic competition to Seroquel, the company’s blockbuster anti-depressant, before December 2012
Is it a mark of desperation? UK-based pharma company AstraZeneca has resorted to suing the US Food and Drug Administration in a bid to stop it from approving generic competition to Seroquel, the company’s blockbuster anti-depressant, before December 2012. The core patent for Seroquel IR expired in September last year, and its paediatric patent runs out this month, although Seroquel XR (the later version) still has protection till 2017.
The FDA has yet to approve a generic version, and AstraZeneca is arguing that it should not do so. The company claims that important decisions have still not been made over warning labels on the generic versions of Seroquel IR, which need to mimic those on the original, while it still has data exclusivity rights stemming from the clinical trials it has conducted.
AstraZeneca tried a Citizens’ Petition against the FDA earlier this month, with no joy, so it is now trying a lawsuit. The case is a long shot, but the costs will certainly be far lower than the losses the company will incur when Seroquel’s patent expires. The drug generated sales of US$4.3bn worldwide last year, of which US$3.3bn was in the US. Moreover, if AstraZeneca wins, then it (and other companies) may also have developed another way of extending protection for many of the drugs that are coming off-patent.
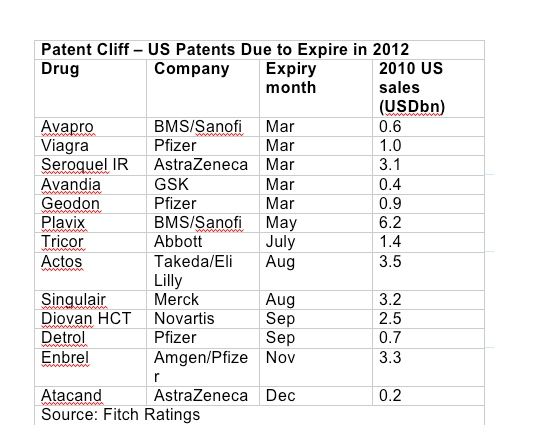
If so, that would be very welcome news in this, the steepest year of the patent cliff. This year, around US$27bn-worth of blockbuster sales will be exposed to generic competition in the US, according to Fitch Ratings. AZ, along with Pfizer and Forest Laboratories of the US, is one of the companies hardest hit. AZ calculates that it lost almost US$2bn in revenue during 2011 as a result of generic competition. Other companies have tried different tactics to protect their patents – Pfizer, for example, struck exclusivity deals with pharmacy benefits managers to protect sales of Lipitor after its US patent expired late last year.
Yet the mood in the US is firmly against pharma companies who are seen as blocking competition, making the likelihood of AZ winning its case appear remote. Though attempts to legislate have foundered, the current US government has campaigned against what it dubs “pay-for-delay” tactics, which mainly involve deals between pharma companies and their potential generic competitors. The chairman of the Federal Trade Council Jon Leibowitz claimed last year that such arrangements cost consumer US$3.5bn a year in higher drug prices.
Addressing Disparities in Psoriasis Trials: Takeda's Strategies for Inclusivity in Clinical Research
April 14th 2025LaShell Robinson, Head of Global Feasibility and Trial Equity at Takeda, speaks about the company's strategies to engage patients in underrepresented populations in its phase III psoriasis trials.
Beyond the Prescription: Pharma's Role in Digital Health Conversations
April 1st 2025Join us for an insightful conversation with Jennifer Harakal, Head of Regulatory Affairs at Canopy Life Sciences, as we unpack the evolving intersection of social media and healthcare decisions. Discover how pharmaceutical companies can navigate regulatory challenges while meaningfully engaging with consumers in digital spaces. Jennifer shares expert strategies for responsible marketing, working with influencers, and creating educational content that bridges the gap between patients and healthcare providers. A must-listen for pharma marketers looking to build trust and compliance in today's social media landscape.
Pfizer, GSK Gain ACIP Recommendations for RSV and Meningococcal Vaccines
April 18th 2025The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted to expand access to Pfizer’s respiratory syncytial virus vaccine Abrysvo for high-risk adults in their 50s and voted in favor of GSK’s meningococcal vaccine, Penmenvy, for streamlined adolescent protection.