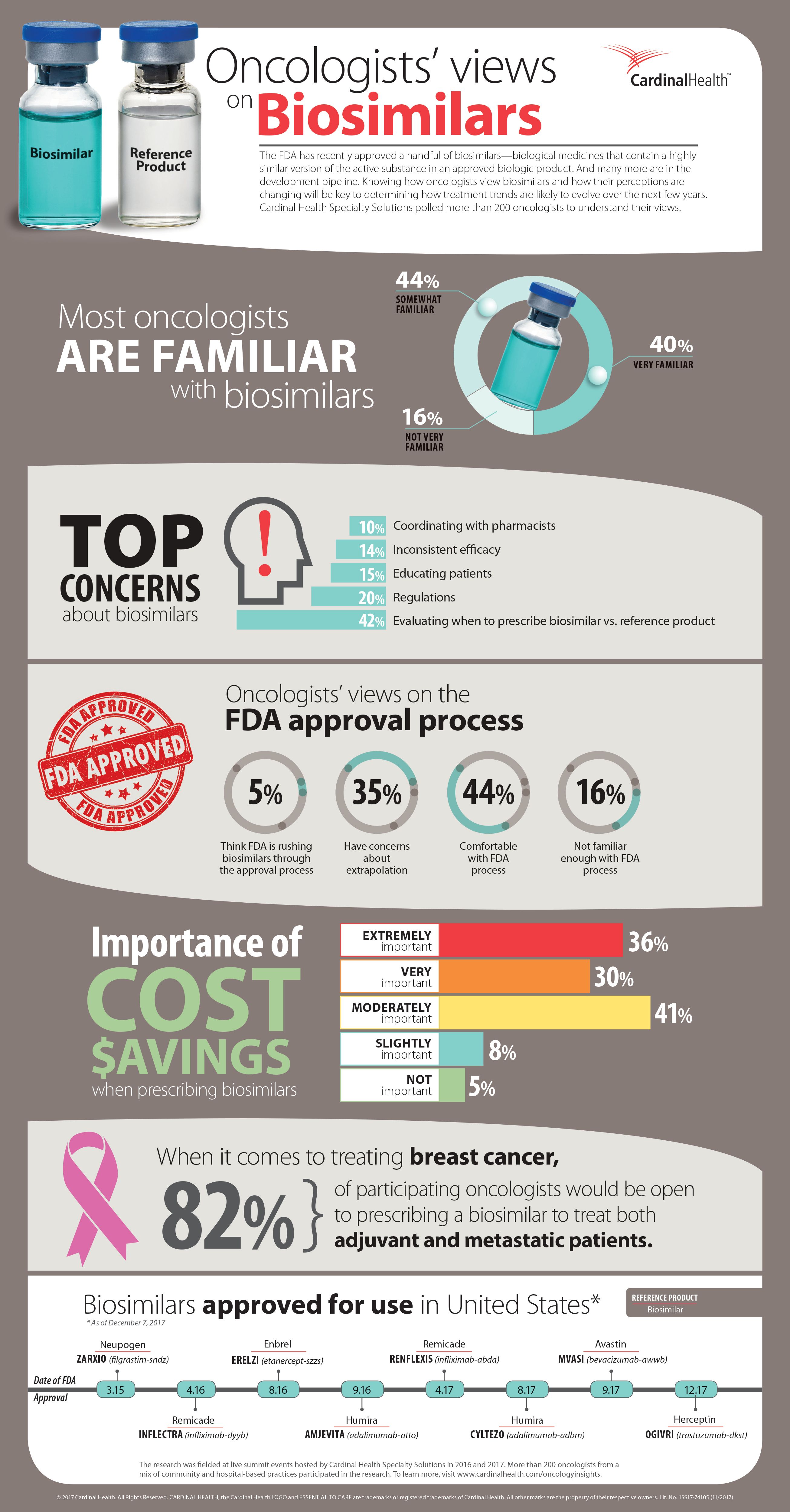
Cardinal Health Explores Oncologists’ Views on Therapies
Cardinal Health Specialty Solutions, healthcare services and products company, released its second edition of Oncology Insights. The research-based report outlines the views of over 200 oncologists in the US pertaining to the latest medical advances and potential for treatments.
The report found that:
- Fifty-one percent of oncologists see CAR T-cell therapy as a game-changing approach to cancer treatment, but barriers such as cost, toxicity and complex administration could lead to slow uptake.
- As more biosimilars enter the U.S. market, oncologists are open to prescribing them in place of their reference products; and 66% have high expectations about the cost savings biosimilars will deliver for their practices.
- With three targeted therapies recently approved for acute myeloid leukemia (AML), most oncologists (85%) are routinely prescribing genetic tests for AML patients – and a growing number (31%) are now referring AML patients to academic medical centers for treatment.
FDA Issues Refusal to File for ImmunityBio’s sBLA for Anktiva in BCG-Unresponsive NMIBC
May 6th 2025The Refusal to File letter comes despite the FDA’s prior encouragement to submit a supplemental biologics license application for Anktiva in patients with BCG-unresponsive non-muscle invasive bladder cancer with papillary disease.
Navigating Distrust: Pharma in the Age of Social Media
February 18th 2025Ian Baer, Founder and CEO of Sooth, discusses how the growing distrust in social media will impact industry marketing strategies and the relationships between pharmaceutical companies and the patients they aim to serve. He also explains dark social, how to combat misinformation, closing the trust gap, and more.