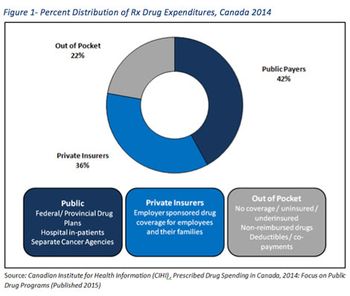
Arvind Mani and Sherry O' Quinn outline the prevalence of private payer PLAs in Canada.
Arvind Mani is the Director of Market Access and Policy Research at PDCI and is responsible for providing strategic advice in the development of reimbursement submission dossiers that help clients demonstrate clinical- and cost-effectiveness to payers and health technology assessment agencies. Arvind has established expertise on emerging market access topics related to product listing agreements (PLAs), biosimilars, and drugs for rare diseases.Sherry O’Quinn is the Director of Reimbursement Strategy, responsible for leading PDCI’s product listing agreement (PLA) services and related strategic reimbursement advice. She brings unparalleled experience and insights into the negotiation of PLAs and the needs of public payers. Sherry has a broad knowledge of the entire pharmaceutical landscape, including experience working in government, industry, hospital, and the retail sector.
Published: February 18th 2016 | Updated: