
A new report from the Tufts Center for Study of Drug Development (CSDD), confirms that companion diagnostics are an important factor for pharma companies to consider when seeking reimbursement of their drugs.

A new report from the Tufts Center for Study of Drug Development (CSDD), confirms that companion diagnostics are an important factor for pharma companies to consider when seeking reimbursement of their drugs.

Global consultancy Cegedim has released its latest survey on how and where big Pharma puts its promotional dollar.

A survey of 600 patient groups worldwide conducted in late 2012 revealed that perceptions of the pharmaceutical industry soured in the past year, mainly due to issues of access, pricing, patient safety and transparency.

The Tufts Center for the Study of Drug Development (CSDD) sees two key trends as increasingly important for pharmaceutical companies to maintain success:

30 years ago last week, the US Orphan Drug Act came into being, and with it, a door of possibilities opened up for patients with literally thousands of untreated diseases.

Thankfully, the civil war is not a public relations issue for Big Pharma, but the long-term business implications for the Middle East region are cagey.

The Securities and Exchange Commission filed charges on Eli Lilly and Co. yesterday for violations of the Foreign Corrupt Practices Act (FCPA) that allege the drug maker’s subsidiaries overseas bribed foreign officials in Russia, China, Brazil and Poland.

At the end of last month, the European Medicines Agency approved its budget and work program for the coming year, promising to implement measures to increase operational efficiency, enhance transparency and communication with stakeholders, and improve the quality and consistency of rulemaking.

The National Intelligence Council’s Global Trends Report explains the scenarios in which the next 18 years may play out internationally.

Yesterday Acura Pharmaceuticals launched its new pseudoephedrine-based product Nexafed, an over-the-counter product aimed at deterring abuse of the illicit drug methamphetamine.

The pharmaceutical industry’s global reputation is on the mend, even if some countries – notably China – are more hostile, according to the latest Ipsos Reputation Snapshot for the Pharmaceutical Sector, from the Ipsos Global Reputation Centre.

The Patient-Centered Outcomes Research Institute, or PCORI, announced last week that it will be granting a total of $12 million for up to 14 contracts for studies aimed at improving upon existing research methodologies to demonstrate clinical effectiveness.

Price Waterhouse Cooper’s “Pharma 2020: From vision to decision” parses a mix of gloomy and glowing scenarios that reflect the fickle, delicate nature of today’s industry.

The pharmaceutical industry as of late has faced a growing movement against patent protection in favor of providing access to low cost medicines, specifically in developing countries.
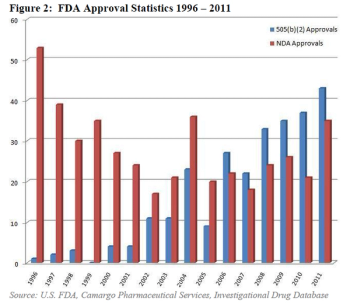
It was in 1984 when FDA first added the 505(b)(2) pathway for drug approval, a hybrid between the accelerated pathway for generic drug applications, and the standard de novo NDA pathway for proprietary drugs.

Featured panelists at ExL’s annual Digital Pharma East conference this week included practicing physicians, vocal ePatients, social media gurus and digital marketing consultants with plenty of hours logged in big pharma boardrooms.