
At the rate science is progressing, there may be more new knowledge in the next 25 years than in the past 100. That's great news, but not if you are planning to run your business as you run it today.


At the rate science is progressing, there may be more new knowledge in the next 25 years than in the past 100. That's great news, but not if you are planning to run your business as you run it today.

Where is pharma going? Toward more science, and more political pressure on science. Toward greater patient responsibility-and more regulation-by-lawsuit. And forward. Let's not forget about forward.

Safety advocates want to make it tougher for new drugs to win FDA approval. Consumer advocates want to make it legal to import drugs from abroad. Put those ideas together, and what have you got?

Understanding how drugs are bought and paid for has always been a bit complicated. People used to say that pharma had two customers-physicians and patients. Only one of them used the drug, and neither of them knew the price. My, how times have changed. Now the industry has so many customers, it needs to stop and get to know them all over again. And that's at a time when drugs worth tens of billions of dollars are going off patent. To map pharma's shifting landscape, Pharm Exec convened a group of top market researchers to discuss the issues shaping an evolving industry. Topics ranged far and wide, from the advent of Medicare Part D, to the new focus on adherence, the role of international markets, even the brave new world of marketing to seniors' children.

The newspapers seem shocked to learn that scientific journals occasionally publish things that aren't true. Where have they been?

Is the public ready for an exciting thriller about terrorism, drug counterfeiting, valiant FDA agents, and glamorous women who invite pharma CEOs to come up and look at their drug patents? Sure. Maybe someone should write one.

Witnessing an industry's wild ride is perhaps the best reason to work for a business magazine. You've given us 25 years of excitement. And we plan to pay you back.

Think of the role compliance plays in your job. Now imagine that level of concern increased by 25 percent, 50, or even more. That's what pharma has to look forward to in the next few years, as the effects of old regulatory initiatives, such as 21 CFR Part 11 and Sarbanes Oxley, start fully kicking in-and as we experience the as-yet-unknown regulatory fallout of the new concern with drug safety. It's no surprise that a great portion of this volume of Pharm Exec's Successful Product Manager's Handbook series is given over to compliance.

If congressmen gathered to work through the nuances of banning ED drugs from reimbursement under Medicare, they'd still back the ban. But the decision would be grounded in lack of faith in prescribing behavior, not disregard for a debilitating condition.

If congressmen gathered to work through the nuances of banning ED drugs from reimbursement under Medicare, they'd still back the ban. But the decision would be grounded in lack of faith in prescribing behavior, not disregard for a debilitating condition.
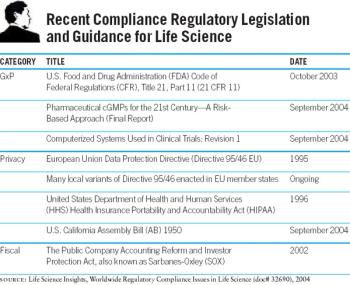
For pharma companies large and small, one of the most pressing challenges of the next few years will be to understand compliance at a much deeper level, to obtain the tools to make it possible.

Part of the value of a drug comes from the supply chain that protects its integrity. There's a similar supply chain that preserves the value of drug information. And it needs help.

A new TV doctor show features the most loathsome pharmaceutical executive in recent memory. But in another important way, the show is serving pharma's best interests.

In a bright airy office above fifth avenue in New York, Lynn O'Connor Vos is talking about topics dear to her heart: trust-and how pharma can regain it-the need to put physicians back in the center of pharmaceutical marketing, and reinvention, a theme in her own life and the core to her approach to business.

Could it be that someone's finally going to wipe the grin off Smiling Bob's face? As we were putting this issue to bed, the Cincinnati newspapers reported that federal agents had raided the offices of Berkeley Premium Nutraceuticals, a company best known for its "natural male enhancement" pill, Enzyte, and for its repulsive television commercials starring Bob. The US Postal Service led the operation, which also included the FBI, IRS, and FDA. They froze bank accounts, sent employees home, and combed records, attempting to determine whether Berkeley, which has accumulated more than 5,000 complaints with the Cincinnati Better Business Bureau and the Ohio attorney general's office since 2001, had committed mail fraud.

Journalists try to tell both sides of a story. That's fine when there are two groups in conflict. But how do you tell the story right when the real conflict is between the two halves of one ambivalent opinion.

Reeve had done something that many of us who communicate about health issues try in vain to accomplish.

In the real world, progress doesn’t result in fewer complaints. It leads to complaining about better things.

What are the industry's current hiring needs? What functions are most challenging to retain? How do misconceptions about working in pharma affect recruiting? These were some of the questions raised at an exclusive roundtable on pharma industry recruiting, co-sponsored by Pharmaceutical Executive and the New York Times Job Market.

College isn't just for students anymore. It's for companies too. Over the past decade, institutions of higher education have increasingly found that some of their most important stakeholders are their students' employers?and that they can extend their reach and influence by responding to the needs of local and regional business.

I suppose, when you get right down to it, I've gotten used to the idea that in this country we don't actually debate issues. We holler and call our political enemies names. We play elaborate games of spin control and do our damnedest to ensure that every question of policy, no matter how straightforward and practical, gets linked in the public mind with abortion rights, gun control, and a half dozen other completely intractable hot-button issues.
When I was a kid, I remember reading magazine articles that set out to determine the worth of the human body-not its personal value, or its ability to produce valuable labor, but just the market value of the chemicals that composed it. The figure I remember hearing was $1.98. Pocket change for the crown of creation.
How should drug companies and physicians interact? And if something is wrong with the relationship, who's to blame?
My train reading this week has been Protecting America's Health, Philip J. Hilts' enlightening history of the Food and Drug Administration. It's a book with a strong sense of how politics, people, and the uncontrollable flow of events conspire to shape institutions. It's also a good read, thanks to the author's fine eye for anecdote. Over and over, Hilts selects just the right story to capture the essence of an era in the agency's history.

In the excellent online magazine The Edge (www.edge.org), Gerd Gigerenzer, director of the Center for Adaptive Behavior and Cognition at the Max Planck Institute for Human Development in Berlin, poses a riddle about risk: Imagine that a 40-year-old woman has her first mammogram and it comes back positive. The incidence of the disease in her age group is 1 percent. The test is 90 percent accurate, and it has a false-positive rate of 9 percent. What's the probability that the woman has cancer?

The trick in understanding any contentious public debate is to figure out what's being left out of the discussion. What, for example, is the assumption that's so obvious that no one thinks to bring it up? More often than not, when you've located that assumption, you've found the secret core of the argument -- the part that everyone's really fighting over and no one can bear to mention.