
Approval of Imfinzi marks the first and only perioperative immunotherapy indicated for muscle-invasive bladder cancer.


Approval of Imfinzi marks the first and only perioperative immunotherapy indicated for muscle-invasive bladder cancer.
As part of the Fast Track designation, Sanofi is set to launch a Phase I/II trial to evaluate the immunogenicity and safety of its novel mRNA vaccine in preventing chlamydia.

Marcel Botha, CEO of 10XBeta, discusses shortcomings in the US's emergency plans for rapid innovation and manufacturing during public health crises, highlighting the need for innovation pipelines, supply chain resilience, and regulatory clarity.

Approval of Vykat XR marks the first treatment indicated for hyperphagia in patients with Prader-Willi syndrome.

Blujepa marks the first new oral antibiotic to gain FDA approval for uncomplicated urinary tract infections in nearly 30 years.
Fanhalta marks the first and only treatment for C3 glomerulopathy to gain FDA approval.

Peter Ax, founder & CEO of UpScriptHealth, discusses the impact of tariffs on the pharmaceutical industry and how digital health platforms could potentially help offset them.

The Project Farma president discusses various ways the tariffs could impact the industry and how businesses can prepare or react.

Dr. Dina Radenkovic, CEO of Gameto, discusses the key considerations for bringing iPSC-based fertility treatments to market from a regulatory and manufacturing perspective.

A look at the current landscape and how young companies can set the stage for product launch.

Jonathon Whitton, AuD, PhD, VP, auditory global program head, Regeneron, discusses regulatory challenges for DB-OTO, Regeneron’s AAV-based gene therapy for hearing loss.

Dr. Dina Radenkovic, CEO of Gameto, discusses the use of iPSCs in fertility treatments, the most significant scientific hurdles they've encountered in developing and scaling these therapies (particularly in relation to ovarian aging), and how they're addressing them.

Dr. Hernan Bazan, CEO of South Rampart Pharma, discusses his company’s current pipeline.

Fred Aslan, MD, CEO, Artiva Biotherapeutics, explains what the FDA Fast Track designation for AlloNK in autoimmune diseases means for the acceleration of its development and potential approval of the therapy.

What biopharma companies need to know about this evolving regulatory pathway.
Omlyclo, a biosimilar to Xolair, is indicated for the treatment of moderate to severe persistent asthma, chronic rhinosinusitis with nasal polyps, immunoglobulin E-mediated food allergies, and chronic spontaneous urticaria.

Jonathon Whitton, AuD, PhD, VP, auditory global program head, Regeneron, discusses how key regulatory designations, such as the FDA's Regenerative Medicine Advanced Therapy designation, facilitate accelerated development and commercialization of DB-OTO through increased collaboration with regulators.

Dr. Hernan Bazan, CEO of South Rampart Pharma, discusses his company’s current pipeline.
The approval of neffy marks the first major advancement in epinephrine delivery for patients over four years of age in more than 35 years.
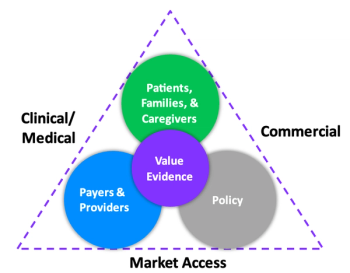
The recent shift in Health Economics and Outcomes Research functions across major pharmaceutical companies highlights a lack of understanding of its value.
Submission is supported by positive results from the Phase III REGENCY trial, which demonstrated that nearly half of patients with lupus nephritis who received Gazyva/Gazyvaro plus standard therapy achieved a complete renal response.

In this Pharmaceutical Executive video interview, Jen Butler, Chief Commercial Officer for Pleio, explores what kind of role regulatory agencies should play in the battle against misinformation.
New indication for Tevimbra in combination with platinum-containing chemotherapy as a first-line treatment addresses an unmet need for adults with unresectable or metastatic esophageal squamous cell carcinoma whose tumors express PD-L1.
Stoboclo, a biosimilar to Prolia, is indicated for postmenopausal women and men at high risk of fracture, while Osenvelt, a biosimilar to Xgeva, is indicated for preventing skeletal-related events in patients with multiple myeloma and bone metastases from solid tumors.

In this Pharmaceutical Executive Video Interview, Peter Ax, Founder & CEO of UpScriptHealth, explores how tariffs could affect the availability and accessibility of specific medications, particularly for patients relying on direct-to-patient telehealth.