The Adherence Journey: Activating the Patient
Pharmaceutical Executive
A patient has been prescribed a drug, your company’s drug. What next? Is there an app for that?Step back and think about the patient journey to this point. In the course of the joys and trials of day-to-day living, a patient may be afflicted with early symptoms. Be they minor or severe, a patient hopefully receives a diagnosis, often after missed opportunities and wrong turns than can stretch for years.
A patient has been prescribed a drug, your company’s drug. What next? Is there an app for that?
Step back and think about the patient journey to this point. In the course of the joys and trials of day-to-day living, a patient may be afflicted with early symptoms. Be they minor or severe, a patient hopefully receives a diagnosis, often after missed opportunities and wrong turns than can stretch for years.
Then, the patient and their caregivers must navigate a maze of bureaucratic networks, cryptic payment terminology, and call centers. And most must do this with limited understanding of their personal physiological perturbation nor of the molecular remedy that has been deemed their savior. Many patients do successfully traverse this path.
Now the patient has your drug (or a slip of paper giving permission to obtain your drug) in hand. The patient can now partake in the great miracle of modern medicinal chemistry. They can begin to reap the benefits of an efficacious and safe therapy, deemed so by years of clinical data.
And yet, many don’t.
Whether they never get the drug, they fail to take it, they miss days, they start but stop because they don’t think it’s working or find a side effect unpleasant, patients fail to adhere at all different stages for countless reasons. Many are just forgetful.
Of course the concept of compliance or adherence is not new. Papers have been written and conferences have been convened for years to get at the heart of why patients don’t follow orders, and what providers, payers, and drugmakers should be doing to improve the state of things.
As it stands, the annual cost of non-adherence to the US healthcare system is estimated at as much as $290 billion. According to a 2014 IMS report (looking at 2012 numbers), non-adherence accounts for about half of the US healthcare system’s “avoidable costs,” dwarfing issues like delayed evidence-based treatment practice, antibiotic misuse, medication errors, and suboptimal generics use.
One clear missive from participants at the BIO International Convention, and at CBI’s PAAS, the 14th Annual Patient Adherence and Access Summit, both held in Philadelphia this summer, is that this remains an untapped opportunity. But the current mindset of “Compliance” and “Adherence” won’t cut it. “Engaging” and “Activating” patients is the new mode of thinking and will be necessary to unlock the potential value. The difference may seem subtle, but a new mentality will be crucial as apps and mobile techs open the door wide to patients’ palms and wrists, hopefully for meaningful interactions that motivates positive behavioral change.
Pharma’s not so adherent programming
From the perspective of drugmakers, solutions to adherence can be elusive. Progress has been made assisting patients with obtaining and staying on their meds. But their commitment to the problem can be described through numerous “fits and starts”, Robert Nauman, principal at BioPharma Advisors Network told Pharm Exec in May.
Major pharma players have hired adherence czars and given the issue attention in two- or three-year spurts, but they’ve never made the radar of mainstream marketing leaders who invested significantly in big brands, he noted.
“Part of the adherence quandary is that these programs are heavily regulated, and as such, they’re not valued as independent programs by the very people they are designed to assist, the patients,” Nauman added. Content produced by adherence teams is often under valued by patients, because of concerns communicating side

effects and adverse events. As programs near launch, drugmakers fear bridging into medical liability issues. Every few years firms look at their adherence programs and ask whether they should be investing in these efforts at all, according to Nauman. “Investments in these tools/programs require resources and commitment, something only specialty pharmaceutical products seem to be willing to do at this time.”
In addition to regulatory constraints, staying steadfast to adherence programs is challenging because it takes work.
“Who’s responsible for adherence?” asked Brian Mullinax, national accounts director, market access, for Flowonix Medical, posing the question to a room full of adherence experts at PAAS in June. “Everyone is responsible and that’s the tough part of the equation,” he said.
Mullinax presented a case study for an adherence program for Genentech’s orphan drug Pulmozyme for cystic fibrosis (CF), an effort to return value for a drug in a challenging setting, which had been plagued by poor adherence. The approach, traditional and simple but comprehensive, was an education initiative encompassing all aspects of CF care. The strategy focused on delivering the message to patients seven times during each quarterly clinic visit via tent cards, posters, teaching handouts, reward program, and verbally with each healthcare provider able to identify the knowledge deficiency and link the adherence message.
The Pulmozyme program was a success, thus meeting goals for prescription, adherence, and clinical measures pulling the product out of a failing trend. But the need to examine and assert the communications strategy for a failing product illustrates the inconsistent adherence approach that Nauman had described, as these measures and the dedicated messaging weren’t fully in place from the start.
Educating a patient population can be a massive effort with the subpar scientific and medical literacy of the lay public trying to comprehend jargon that can confuse and intimidate patients. Thomas Bauer, corporate director of health literacy and patient engagement for Novant Health, pointed to one of the most basic (and dramatic) events in medical communications, the moment when a patient receives test result which can be “positive” and be bad, while “negative” means good-a language obstacle so fundamental that this writer can think of multiple times it’s been used as a comedic device in TV sitcoms (Seinfeld).
Though the straightforward, traditional adherence effort produced results for Pulmozyme, communicators across all walks of life can attest to a basic frustration-the deficit model. Simply put, scientific and medical professionals assume that imparting knowledge and giving a greater understanding will result in altered behavior. But for whatever reason, knowledge doesn’t always result in a transformed patient.
The thinking of adherence programs is flawed because they are product-centric, noted Megha Reddy, manager, patient engagement for GlaxoSmithKline. Adherence
Megha Reddy
programs are missing an important aspect of the patient experience, she explained. Knowledge is power and patient education is a big focus, but the patient’s ability to manage their condition is still limited. Knowledge is really just a part of the equation. Motivation and skills are needed, too. To this end, GSK has focused on influencing behavioral change using 50 years worth of behavioral science research.
Reddy presented examples where GSK has transformed traditional patient resources into more engaging experiences for patients. The example given at PAAS, rather than informational brochures for smoking cessation, GSK presents patients with interactive, informative, and instructional teaching platforms-more like choose your own adventure guides than static textbooks. These have helped patients actively build the motivation and skills to quit smoking, rather than just passively being told what to do, she explained.
So drugmakers, payers, and healthcare providers can, and should go to great lengths to educate patients about their disease and treatment options. Simple is key, and adherence starts at the clinic, noted Mullinax. But simply giving information and instructions from a position of authority without engaging patients can be inadequate and can backfire. This is why those in the field are excited to take thinking on adherence in a new direction, towards patient activation.
Does a change to an “activation” mindset really mean anything? Or is this just the newest buzzword for lunchroom corkboards and outward facing corporate PowerPoint’s that will be replaced in five years?
Regardless of what patient support will look like in five or 10 years, right now, the change certainly appears to be on. At PAAS, it certainly seemed like attendees who hadn’t already done so, would be going back to the office and running a Find/Replace on all corporate documents to scrub out “compliance” and “adherence” in favor of “activation.”
Words to deed
According to Alisa Hughley, of enBloom Media, speaking on a panel at PAAS, patients are demanding the change because they are becoming proactive, rather than being acted upon. The mindset of adherence and compliance makes taking a medication an act of submission, she explained. Patients, especially those with a chronic condition, are already submitting to the idea that they have this condition and have to learn to live with it.
“The notion that a physician can just give instructions and you’ll be fine is archaic,” added co-panel speaker Katherine Leon of the SCAD (Spontaneous Coronary Artery Dissection) Alliance, calling the traditional adherence mindset almost militaristic. “A safer approach would involve patient and doctor taking the time to fully discuss all medications.”
Kimmia Forouzesh, of the Foundation for Sarcoidosis Research, noted that her organization has also preferred a different mindset, one of “empowering” patients. In sarcoidosis, the treatment plan varies by patient as there is (as of yet) no drug developed to treat the underlying cause of the disease, so patient empowerment is crucial, she noted.
Solidifying the point, Hughley quoted participants of a recent focus group:

“I hate, hate, hate the word adherence! It makes patients who can’t follow instructions to the letter sound like unruly children.” And, “Adherence implies a level of obeying. It doesn’t feel like the patients and caregivers are partaking in a collaborative effort.”
“Words have power,” Hughley added. “It’s not about getting a patient to adhere. It’s about engaging patients in shared clinical decision-making.”
Wellness-the deep stream
More than a change of mentality for drugmakers and caretakers to have towards patients, many see the prospect to meet patients in a collaborative way as a huge opportunity with mobile tech and apps as key mediums for connecting.
On another panel discussion just a week prior to PAAS, the Scientific American Worldview Super Session at the BIO International Convention, moderator David Brancaccio of the Marketplace Morning Report and PBS Now, asked his esteemed panelist what they see as this generation’s “plastics,” in reference to the famous advice given to Dustin Hoffman’s character, Ben Braddock, in The Graduate.
Lee Hood, President Institute for Systems Biology, deemed “wellness” as the next “plastics,” pointing out that currently 99% of spending is on disease and just 1% is put towards wellness, but this will change. Hood noted that a key step will be making the study of wellness more scientific. Tech-enabled, activated patient populations should make health and wellness trials possible for attaining true scientific results. Hood recently co-founded Arivale, a Seattle-based firm based on the very idea of scientific wellness which promises to utilize a 360-degree view of its consumers’ DNA, blood and saliva, gut microbiome, and lifestyle.
Echoing Hood’s message, Martin Naley, founder and CEO of Cure Forward, said “patient activation” was his 2015 substitute for “plastics.” The notion of patients getting activated by their own data will be a huge opportunity, he said. Whether patients are visualizing their genomes or counting their steps, we will see a more engaged patient population.
If the prognosticators are correct, investment in patient engagement and activation platforms may be a major trend. But apps and mobile technology evolve at rates that leave pharma and biotechs choking on their dust. Could pharma exit the adherence/activation game all together and just outsource it?
“Disruptive innovators in mobile and tech may disintermediate drugmakers from adherence efforts,” noted Nauman, prior to the PAAS conference. “Diabetes or blood pressure monitoring apps, using gadgets like the Apple Watch, for example-none of these techs are regulated, and innovation is coming at rates that are infinitely faster than pharma can play at,” he said. Patient monitoring is going to be something the technologists take on, and as a result pharma may just forgo big patient support programs. Yet following the conference, Nauman noted: “Even mHealth application developers do not want to wade into the water where some tool can help with diagnosis and medication management to track adherence over time for fear it might be considered a medical device.”
Biogen’s Fitbit trial
Indicating how pharma and biotech companies might manage to keep their skin in the patient adherence and activation enterprise, Biogen has taken major steps to learn how they might just bring patients, drugs, and technology together.
For a start, the company thought it would give Fitbit a chance in a group of email responsive, tech-enabled multiple sclerosis (MS) patients. Director of new initiatives for Biogen’s Innovation Hub, Jane Rhodes explained that the company wanted to try out a commercially available device, employ it to patients, and to simply address whether they would use it and allow Biogen and PatientsLikeMe to access the data.
For something so simple, it was quite complicated to begin with, but Biogen ended up choosing Fitbit because it was commercially available and it has an open application program interface, so it was easy to extract data. The company constructed an “out of the box” experience, which was followed up with interaction to follow patients if they had questions.
Fitbits famously have shown strong sale sales and good early adoption, with a large number of customers falling off, disinterested in a matter of months. In the medical setting, if it’s viewed as valuable and the physicians stress the importance, patients will tend to wear the devices, showing high compliance, Rhodes noted.
The trial was “fantastically successful” with a high number of responders and active patients all the way to a completed survey. High participation was evident as most patients were interested and able to use the Fitbit. The data will be helpful, because not just walking, but sleep, is very important for MS patients.
Of course, this early work was not rigorous enough to show decisive clinical measures, but Rhodes emphasized that the levels of ambulation collected correlated nicely with self-reported standards indicating the strong potential for further use to provide data points in MS trials.
Besides Fitbit, Biogen’s new initiatives director is bringing in other mobile technologies in for its MS mission. Probably the most mature collaboration, the firm is working with the Cleveland Clinic to develop and iPad-based tool designed to be used in the clinic setting to allow patients to conduct their own neurologic testing. “We think it’s a huge step forward for a number of reasons,” says Rhodes. “Patients only see neurologists a couple times a year and most of the appointment is conversational to try to understand symptom progression, new symptoms and their response to treatments.” Biogen and the Cleveland Clinic have take FDA accepted tests for assessing progression quantitatively and put them into an iPad format without impacting the flow of the patient’s care.
Speaking to the impact of mobile techs and the impact they will have on patient support and engagement, “My sense is that we’ve reached a tipping point, and there’s going to be an enormous explosion in techs that can gain traction and transform care,” says Rhodes. “There’s been explosion in consumer space, but it hasn’t helped much in healthcare yet. In terms of transforming care in delivery, we’ve only just scratched the surface.”
And Rhodes agreed that biopharma companies will have to partner with technology experts to tackle the mobile patient. “We live in a world of partnerships now. No one can do this alone.”
Adherent bites
As a prelude to who some of these disruptors and partners just might be, PAAS attendees listened to rapid fire, elevator-style pitches from several tech startups taking Shark Tank-inspired questions, no doubt indicating that adherence-dedicated professionals are on the cusp of tech and TV trends (see sidebar below).
The audience was treated to an array of demos and presentations, including CaseTrack360 from C3i Healthcare Connections, an IT
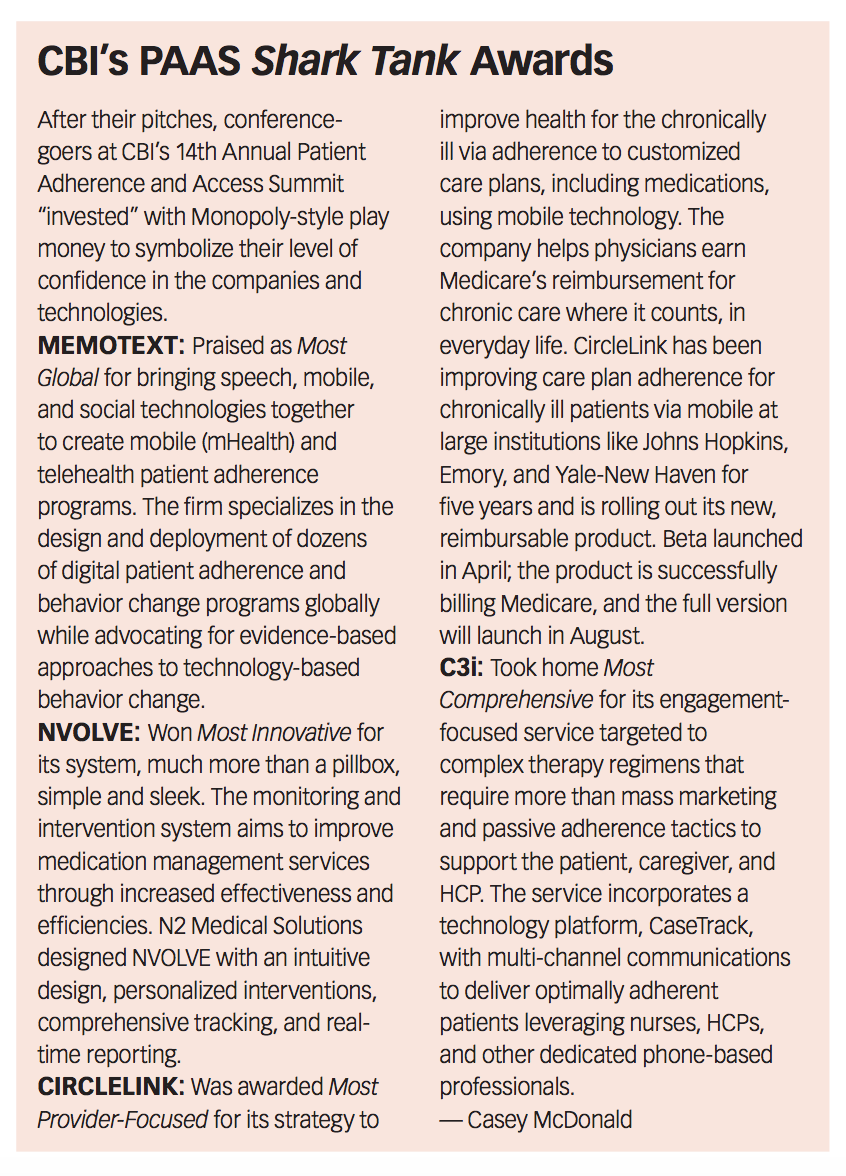
solution for 360-degree patient path monitoring, Memotext's text message-based adherence programming for a patient-specific, personalized interaction platform, and CircleLink Health's mobile phone engagement technology designed for chronic care allowing their physicians to easily earn Medicare’s new chronic care reimbursement.
The most intriguing presenter just might have had the simplest and most unassuming technology for aiding patient medication adherence. Andy Bowline, CEO and co-founder of N2 Medical Solutions, presented the iPhone of pill dispensers called nvolve. Designed for the aging patient, nvolve is simply a pillbox device with weight-sensitive sensors providing a dashboard for the physician to monitor and make dosing decisions. It’s designed for patients who haven’t graduated to assisted living yet and want to maintain independence. The aid, like so many good technologies, can do its job and fade into the background. A device that “fades into the background” may not be in line with the activation theme of the conference, but the questions from the audience certainly indicated the audience’s sense that the device’s simplicity and functionality would be highly sought after.
Actively forward
One thing was clear, PAAS, the Patient Adherence and Access Summit, might just have to change its name. Though, luckily, changing adherence to activation, the CBI event can maintain its acronym.
But what was also clear from a June of adherence discussions, there is immense excitement and massive opportunity for a changing mindset to work with patients engaging and activating them. The potential for tech and mobile solutions to impact patient care rapidly is real, but the regulatory system, along with keeping an eye on real privacy concerns, will have to keep up.
The stakes are high, clearly as high as $290 billion, and that number can only grow with an aging population and increasing specialty pharma spending. With cures hitting markets, and $1,000 per-day pills impacting payers and care networks, the potential impact of patients being able to remember their pill regimens means the pressure for more patient activation will only increase.
Casey McDonald is Pharm Exec's Senior Editor. He can be reached cmcdonald@advanstar.com.

Addressing Disparities in Psoriasis Trials: Takeda's Strategies for Inclusivity in Clinical Research
April 14th 2025LaShell Robinson, Head of Global Feasibility and Trial Equity at Takeda, speaks about the company's strategies to engage patients in underrepresented populations in its phase III psoriasis trials.