Backpage: Doctors with Attitudes
Pharmaceutical Executive
Accredited CME providers place a lot of value on understanding doctors' preferences and opinions. To better educate themselves, providers perform ongoing needs assessments to determine educational requirements, and actively survey clinicians for feedback on industry hot topics.
Accredited CME providers place a lot of value on understanding doctors' preferences and opinions. To better educate themselves, providers perform ongoing needs assessments to determine educational requirements, and actively survey clinicians for feedback on industry hot topics.

Between September and October 2005, CME surveyed attendees at various meetings to get their opinions on issues and trends facing the healthcare industry today. A survey was also conducted to determine the value of educational products.
Healthcare professionals were questioned about the Office of Inspector General (OIG) compliance program for pharmaceutical manufacturers, the implementation of outcomes measurement studies as part of CME, and the value of editorial supplements.
Outcomes measurement Sixty-three percent of psychiatrists agree that outcomes measurement studies should be an integral part of CME activities.
Fifty-two percent of psychiatrists view the implementation of pre-, post-, and follow-up evaluations as a beneficial opportunity to help shape CME.
OIG's compliance program Forty percent of psychiatrists and 28 percent of primary care physicians stated that they are aware of the compliance program.
Sixty-one percent of psychiatrists and 54 percent of primary care physicians believe that OIG's regulation will ensure the objectivity of CME activities supported by grants from the pharmaceutical industry.
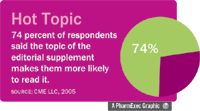
Editorial supplements Seventy-four percent said they are more likely to read a supplement if the topic interests them, while 35 percent said they were most attracted by the supplements' authors.
Hot Topic
When asked if the information provided in supplements ever changed their diagnostic decisions, 65 percent of respondents said yes. Seventy-five percent said supplement information has changed their therapeutic approaches. And when asked if the information published helped them provide better treatment to their patients, 84 percent of respondents said yes.
Barbara Winkelman is vice president of marketing and multimedia for CME. She can be reached at barbara.winkelman@CMELLC.com
Addressing Disparities in Psoriasis Trials: Takeda's Strategies for Inclusivity in Clinical Research
April 14th 2025LaShell Robinson, Head of Global Feasibility and Trial Equity at Takeda, speaks about the company's strategies to engage patients in underrepresented populations in its phase III psoriasis trials.
Beyond the Prescription: Pharma's Role in Digital Health Conversations
April 1st 2025Join us for an insightful conversation with Jennifer Harakal, Head of Regulatory Affairs at Canopy Life Sciences, as we unpack the evolving intersection of social media and healthcare decisions. Discover how pharmaceutical companies can navigate regulatory challenges while meaningfully engaging with consumers in digital spaces. Jennifer shares expert strategies for responsible marketing, working with influencers, and creating educational content that bridges the gap between patients and healthcare providers. A must-listen for pharma marketers looking to build trust and compliance in today's social media landscape.
Pfizer, GSK Gain ACIP Recommendations for RSV and Meningococcal Vaccines
April 18th 2025The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted to expand access to Pfizer’s respiratory syncytial virus vaccine Abrysvo for high-risk adults in their 50s and voted in favor of GSK’s meningococcal vaccine, Penmenvy, for streamlined adolescent protection.
