Benchmarking AIDS
Nothing reflects pharma's response to public-health crises in sharper detail than the industry's efforts to fight the AIDS epidemic.
Nothing reflects pharma's response to public-health crises in sharper detail than the industry's efforts to fight the AIDS epidemic.
With more than 40 million patients infected worldwide—most of them poor residents of countries with underdeveloped healthcare systems—the call to action has never been louder. Drug companies are expected to take bold, creative steps to develop new medicines and ensure access to existing ones. If they do not, they not only fail in society's eyes, they endanger the system that finances innovation and protects intellectual property.
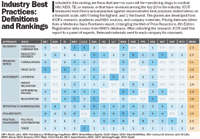
The Interfaith Center on Corporate Responsibility (ICCR), a nonprofit group of faith-based institutional investors, defined best practices for access to drugs in the developing world, based on a consensus of government, NGO, and industry sources. (For definitions of these best practices.) To help companies—and socially conscious investors—understand where pharma's major players stand in relation to these best practices, ICCR compiled a report to benchmark pharma companies' individual responses to the AIDS epidemic and a host of other neglected diseases.
Each company has its own story, to be sure, but several trends emerge from the data. Pharma companies across the board have implemented differential pricing—charging lower prices in less developed parts of the world. Philanthropy is another broad strength of the industry's AIDS strategy.
On the other hand, most companies deal poorly with the epidemic outside of Africa. Few have been willing to relax patents in hard-hit countries that need better access to drugs. And although American firms are better than their European counterparts,companies obscure the relationship between political contributions and their public position on developing-world issues.
The ICCR report compares companies head-to-head to show, for instance, how Gilead spearheads R&D for fixed-dose combinations, but has been hesitant to issue voluntary licenses, while GSK licenses enthusiastically, but lags in fixed-dose combinations. Executives should first take time to understand the rankings. Then, they can dig into the data, which reveals the companies that are moving the needle by transforming theories of best practice into reality.
The Interfaith Center on Corporate Responsibility defines best practices for all of the following aspects of company policy, from research to pediatric treatment. Together, they represent a formula for access to drugs in the developing world.
Research
• Fixed-Dose Combinations (FDCs)
Company is taking a lead role in development or production of FDCs with other companies.
• Neglected Diseases
Company has robust programs to research and develop drugs for a range of neglected diseases.
Pediatric Needs
• Formulations
Company produces a range of child-friendly formulations for each age group throughout its entire clinically appropriate product line.
• Price Cuts
Pediatric treatment costs are equivalent to adult treatment costs.
Accessibility
• Licensing & Technology Transfer
Company has issued three or more non-exclusive licenses for its full range of products, allowing for sales in a wide range of markets. The company provides training and technology to licensees and encourages co-formulation with other brands to develop appropriate fixed-dose combinations.
• Patent Enforcement Relaxation
The company has no patents in countries that are major generic exporters or in Least Developed Countries (LCDs).
• Differential Pricing
Low-income and middle-income country prices are affordable and predictable.
• Registration
Company has obtained registration for all dosages and formulations in all relevant markets. (ICCR gives companies the benefit of the doubt on this topic, and assumes registration except where specific information is available to the contrary.)
Reporting To Shareholders
Company's reporting includes articulation of the business case for action, an assessment of the options for action, systematic reporting of goals and activities, and evidence of board-level leadership. The report also has pricing schemes and timetables for access goals.
Sustainable Philanthropy
Company's philanthropic programs are well integrated into its overall access-to-medicines programs. They are wide-reaching and sustainable. The programs are built into the company's business strategy and reported to shareholders as such. The activities and impacts of the programs are continuously monitored.
Political Engagement
• Political Contributions
The pharmaceutical company reports on all political contributions, providing individual rationales for each candidate and group to whom it contributes. The company has board oversight of political contributions.
• Trade Associations
Company fully discloses its trade association dues and payments to third-party organizations, as well as how those dues are directed. Its dues and payments are consistent with its public position on public-health issues. Or, the company is not a member of a pharmaceutical or other trade organization so does not need to report separately on dues.
[Norvir Stands Alone]
Abbott's lack of collaboration with generic or other branded companies is troubling—particularly because a number of other ARVs must be taken with Norvir. Abbott also takes a hard line against issuing voluntary, non-exclusive licenses—most famously against Brazil—and maintains, "Abbott's HIV patents are not preventing access to HIV treatment in developing countries."

Abbott says it makes its HIV drugs available throughout Africa, but some NGOs have complained that the drugs aren't registered or available in other markets. In particular, MSF China has been contacting Abbott since June 2004 about the "growing and urgent" need for access to second-line drugs, such as Kaletra. To date, Abbott has not yet registered the new, improved, heat-stable version of Kaletra in any developing country, stating that it is first waiting for EU approval. This is a key second-line drug, which Abbott must ensure is universally registered, available, and affordable in adult and pediatric formulations.
As for research, there is an urgent clinical need for a number of products Abbott could provide: improved pediatric formulations (that maintain Abbott's already commendable pediatric pricing), heat-stable Norvir, additional FDCs containing Norvir, and low-cost generic lopinavir+ritonavir.
[No Free Lunch ]
Boehringer-Ingelheim is perhaps best known for its Viramune Donation Program, through which it supplies free nevirapine to treat mother-to-child transmission of AIDS. However, the program remains controversial—critics argue that by giving away the drug for MTCT programs, the company undermines the generic market for nevirapine, complicates procurement systems, and even contributes to stigma by distinguishing between "innocent" newborns and other patients.

The company considered combining Viramune and Combivir (lamivudine+zidovudine) but concluded that FDCs "turn into a regulatory nightmare," and that it doesn't know "how to make it happen, given the anti-trust laws." Instead, BI and GSK co-packaged Viramune and Combivir, and licensed it to Aspen Pharmacare to distribute in Africa. However, the manufacturer should resolve its FDC issues, since Aptivus (tipranavir), an AIDS drug for resistant patients, must be co-administered with Norvir. It also ready to ship continue to research pediatric AIDS formulations—but gains top ranking for its pricing strategies on child dosages.
[Focus on TB]
Given its size, the scope of AstraZeneca's developing-country philanthropy and neglected-disease research efforts lags behind competitors. However, while the company doesn't study AIDS drugs, it is developing a TB treatment in its Bangalore, India facility, and has the potential to make a significant contribution to finding new treatments for tuberculosis.

[Partnership Breeds First FDC]
BMS shows flexibility on licensing and patents, continues to make significant research commitments in HIV/AIDS (but not other areas of neglected diseases), and creates effective partnerships. In particular, BMS, Gilead Sciences, and Merck have created the first branded FDC Atripla (efavirenz/tenofovir/emtricitabine). More is needed: Reyataz is considered especially effective when used with Abbott's ritonavir.
The royalty-free voluntary licenses and technology transfers for Reyataz with Aspen Pharmacare and India's Emcure came less than two years after the US launch of the drug, but BMS needs more licensees to comply fully with best practice.

BMS' core need is more effective reporting, with quantitative goals. For example, BMS hired the International Association of Physicians in AIDS Care to evaluate its philanthropic Secure the Future program in 2001. But no new full-scale evaluation has been completed. The lack of information on the accessibility of BMS' new FDC, including pricing schemes, registration efforts, and timelines for new pediatric formulations, must be addressed.
[Wanted: Drugs for Kids]
Gilead is a nimble company that has helped develop the new FDC Atripla. But to overcome criticism, it must focus on pediatric formulations and drug registration in the developing world.
An oral pediatric solution of Emtriva is not scheduled to be available in developing countries. Gilead is not yet marketing pediatric formulations of Viread or Atripla.The company has not yet researched low-dose or scored tablets.

Gilead is strong on patent rights, and has applied for a patent of tenofovir in India under the country's new laws. In a major challenge to the new Indian legislation, advocacy groups oppose the application, which gives Gilead 12 years of exclusivity.
Gilead does not disclose political contributions. That is particularly problematic because it has high-profile political figures as current (George Schultz) or former (Donald Rumsfeld) directors.
[Research Heavyweight]
GSK is an industry leader in reporting, licensing, and research. It has the most substantial neglected-disease research program in the industry, with 13 clinical programs targeting eight diseases relevant to developing countries, and has recently brought to market Rotarix vaccine, which treats severe diarrhea in infants.
GSK takes a pro-patent stance, and currently is seeking a patent in India for Combivir—the first ARV to be reviewed under India's new patent laws. GSK also has a monopoly on Epivir in China, but has not made the drug available in the dosages required.

The company says "having a policy that states that patents will not be enforced in certain markets does not necessarily make it easier for generics to become available. In fact, blanket 'relaxations' could mean that companies 'wash their hands' of the issue and do not register new products...or provide technology transfer."
[Promising Pipeline]
Tibotec, a subsidiary of Johnson & Johnson, is bringing to market several novel HIV therapies and a multi-drug-resistant TB product, which would be the first one in several decades. Although the products have not yet been approved, Tibotec is off to a good start, expressing an openness to partnering with generic companies and granting a royalty-free license to the International Partnership for Microbicides.

J&J reports to the public with a Web site that integrates charitable grants, research, IP licenses, and workplace programs. This is superior to the typical philanthropy-driven reporting at peer companies. Nonetheless, a more explicit discussion of core business risk, especially given J&J's dual exposure to AIDS through its substantial consumer-product operations and sector-specific pharmaceuticals, is necessary.
[Technology Transfer]
Lilly's multi-drug-resistant TB program is a bright spot in the world of tuberculosis treatment. Lilly has taken the extraordinary step of transferring manufacturing technology needed to produce cycloserine and capreomycin to several generic firms. These drugs for MDR-TB no longer have patent protection and so no actual license was necessary. Nonetheless, Lilly made a $70-million technology transfer and support commitment, and matched it with academic and NGO partnerships that increase the capacity of health systems to treat MDR-TB. We do not believe there would be significant generic production of these products without Lilly's engagement. Unfortunately, given Lilly's research priorities and clinical strengths, as well as its lack of an internal neglected-disease program, the company is unlikely to yield new drugs to combat diseases of poverty.

[Philanthropy Know-How]
Merck's history of responding to neglected diseases is strong, and its philanthropy rates the highest in the industry. The company's 20-year-old Mectizan donation program for river blindness is often viewed as a model for drug-donation programs. More recently, Merck has entered into several HIV/AIDS public-private partnerships in Botswana, China, and Romania, in which it has been noted for its significant funding and strong emphasis on treatment and evidence-based prevention programs. However, the company's HIV/AIDS response appears to be overly driven by philanthropy. Merck should tap into the full force of its core business strengths to overcome registration lags—the 600 mg and 200 mg formulations are still only registered in 25 of the 40 Sub-Saharan countries—and a still-tentative licensing approach.

To date, Merck has issued a license to Aspen Pharmacare, and also to Thembalami, a joint venture of Adcock and Ranbaxy. But when Thembalami dissolved, observers expected Merck to issue the two parent companies licenses—that has not happened. Merck will be under increasing pressure to issue voluntary licenses because several generic companies recently lowered prices on efavirenz in an agreement with the Clinton Foundation. Making those price cuts widely available may require additional licenses.
[Drug Discovery]
Novartis may make a significant contribution to the fight against neglected diseases with its Novartis Institute for Tropical Diseases (NITD), a public-private drug-discovery partnership between Novartis and the Singapore Economic Development Board, which is aimed at major tropical diseases. Other areas of research score high as well: It played a leading role in developing antimalarial FDCs, including Coartem, and has developed two of the three products in the multi-drug therapy used in WHO's strategy to eliminate leprosy.

Novartis is committed to providing the leprosy treatment for free, and is planning no-profit pricing for drugs resulting from NITD research. However, it is unclear if NITD-developed drugs will be licensed, since the research is still in early stages. But, given the promise of this venture, the company should explore licensing and technology transfer as tools for developing essential patented medicines beyond ARVs.
[Industry Leader?]
Pfizer has immense resources which could bring research-based and market-based solutions to bear on the issue of medicine access. However, the firm is heavily dependent on traditional philanthropic approaches to health problems and conducts little research for the developing world. Given Pfizer's disproportionate influence on the industry, these issues are especially troubling.

Pfizer's most notable program is its Diflucan donation program. Pfizer now gives the anti-fungal medication to 21 countries with an HIV prevalence above one percent without a time limit on the donation. However, critics argue that differential pricing and licensing would have been superior alternatives. The Diflucan effort specifically has been criticized for its red tape, narrow focus, and insufficient geographic reach. The company also has employee-volunteer programs and supports healthcare infrastructure development in Uganda. There are no further neglected-disease drugs in the company pipeline. However, Pfizer clearly sees a core business case for action, since it has devoted immense PR resources to addressing issues surrounding access to medicine in the developing world.
[Patent Prowess]
Roche manufactures several ARVs, and is heavily invested in HIV research. However, it has not worked with other companies to create FDCs—particularly for Fortovase, which should be taken with Norvir. The company has even stayed away from blister packaging its products, citing IP and liability issues. It also produces other drugs, like Fuzeon, the first fusion inhibitor—a twice-daily subcutaneous injection that costs $20,000 per year—which is not even remotely accessible to patients in the developing world.

Roche has clear patent policies, and has pledged not to file patents on ARVs or malaria drugs in the least-developed countries. Recently, Roche announced a technology-transfer initiative to provide local manufacturers with the technical expertise to produce Invirase, a WHO-recommended treatement.
Roche must also be commended for its predictable, transparent pricing in both LDC and middle-income countries. However, some critics express concern that prices remain way too high in middle-income countries, and that the disparity between pediatric and adult formulations is too great.
[Sustainable Approach]
Sanofi-Aventis leads the industry in its research investment for neglected diseases, with a large number of pipeline products—as well as key essential drugs already on the market for malaria and TB, among other neglected diseases. Currently, the company's vaccines unit is attempting to combine diphtheria, tetanus, pertussis, polio, Haemophilus influenzae type b, and hepatitis B vaccines in a single injection. Through public-private partnerships, it aims to develop an HIV vaccine, and it is running trials for a dengue fever vaccine. An antimeningococcal vaccine will be available in 2006.

The company takes a business-like approach to the developing world, seeing market opportunities in addition to traditional philanthropy. Nonetheless, quantitative metrics would make that reporting more robust.
[Missed Opportunity]
Wyeth is not leveraging its established vaccine business to benefit the public-health crisis. While vaccine research remains a bright spot, Wyeth's other public-health initiatives appear modest.

Today, the company runs trials for a children's pneumococcal conjugate vaccine in Africa and works closely with the Global Alliance for Vaccines and Immunization. It also has a partnership with the Centers for Disease Control to investigate HIV vaccine candidates. But it devotes no substantial resources to public health in developing countries.
Wyeth philanthropy includes product donations but falls short of the enduring, large-scale financial commitments of other companies.
[New Kid on the Block]
Schering-Plough currently has a CCR5 receptor antagonist called vicriviroc in Phase II trials, which may become a second-line therapy. It is exciting to see a new company in this area.

But, access remains a question. The company has made no public statements about exploring licensing options. However, CEO Fred Hassan led Pharmacia when that company out-licensed an ARV and advocated strongly for other companies to do the same.
Most companies would benefit from increasing the quality of their reporting, but for Schering-Plough, which is developing its first HIV drug, this is even more critical. SP has almost no information on the Web or in its annual report concerning its strategy for addressing global health issues.
Conclusion
Ultimately, the soundest risk-reduction strategy for any company is a response that privileges public health, so that the underlying cause of the crisis—infectious diseases in developing countries—is addressed. Although it is not possible for pharma companies to wholly bring this about, there is a wide range of options available that they are failing to take advantage of.
Kieran Hartsough khartsough@iccr.org is a public health research associate at ICCR. Daniel Rosan drosan@iccr.org, is program director for public health at ICCR. Lisa Sachs lsachs@iccr.org is a public health research associate at ICCR.

Addressing Disparities in Psoriasis Trials: Takeda's Strategies for Inclusivity in Clinical Research
April 14th 2025LaShell Robinson, Head of Global Feasibility and Trial Equity at Takeda, speaks about the company's strategies to engage patients in underrepresented populations in its phase III psoriasis trials.
Key Findings of the NIAGARA and HIMALAYA Trials
November 8th 2024In this episode of the Pharmaceutical Executive podcast, Shubh Goel, head of immuno-oncology, gastrointestinal tumors, US oncology business unit, AstraZeneca, discusses the findings of the NIAGARA trial in bladder cancer and the significance of the five-year overall survival data from the HIMALAYA trial, particularly the long-term efficacy of the STRIDE regimen for unresectable liver cancer.
Expanding Immune Response Testing to Support Vaccine Development
April 22nd 2025Nigel McCracken, chief operating officer, Virax Biolabs, discusses the expansion of its ViraxImmune platform into areas such as transplant monitoring, vaccine efficacy, latent virus reactivation, and CAR T cell therapy.