Country Report: Brazil
In its ambition to further strengthen the nation’s relationship with the global industry, Brazil holds a valuable, eye-catching asset: the dynamism of its huge pharmaceutical market, the sixth-largest in the world.
This sponsored supplement was produced by Focus Reports
Report Director: Laurent Pichotzki-Libano
Report Coordinator: Anamarija Svedrec
Report Assistants: Carole-Anne Bruneau, Luca Nardini, Alan Le Roux
Report Publisher: Mariuca Georgescu
Senior Editor: Louis Haynes
Editor: Patrick Burton
Graphic Assistance: Miriam León
Cover photo © Sao Paulo skyline
For exclusive interviews and more info, please log onto
www.pharmaboardroom.com or write to contact@focusreports.net
“Brazil undoubtedly holds great development opportunities for the global pharmaceutical and healthcare industries,” introduces Minister of Health Ricardo Barros, “and we hope to gain the trust of an increasing number of international investors and jointly work on improving the healthcare products and services available to our 207 million Brazilian citizens.” In its ambition to further strengthen a win-win relationship with the global industry, Brazil holds a precious, eye-catching asset: the remarkable dynamism of its huge pharmaceutical market. “Very few of the world’s largest pharmaceutical markets have been growing at a sustained pace over the past years, and Brazil proudly stands as one of the best performing markets,” highlights Jarbas Barbosa da Silva, director-president of the Brazilian Health Regulatory Agency (ANVISA).
“As the second-largest emerging market for Sanofi globally, Brazil is one of the ‘Must Wins’ in the company’s portfolio. It is by no means an easy market, but it is unequivocally a highly attractive market,” adds Pius Hornstein, country chair and general manager Pharma of Sanofi Brazil, the largest international company operating in the country. “Brazil today stands as the sixth largest pharmaceutical market in the world, and recent forecasts suggest that it will likely reach the fi fth position in the coming years; naturally, this sheer market size has cemented the crucial importance of the country in the overall operations of all pharmaceutical companies with global ambitions,” confi rms Rolf Hoenger, president and general manager of Roche Pharma Brazil, the largest player in Brazil’s non-retail market in 2017.

AN INDUSTRY ON A HIGH...
To whoever has been following, even from afar, Brazil's economic and political developments over the past four years, the enduring strength and ever-increasing significance of the local pharmaceutical market might seem surprising, as the country just experienced its worst recession in modern history, coupled with a highly mediatized political crisis that resulted in the impeachment of president Dilma Rousseff and widespread dissatisfaction with the political system.
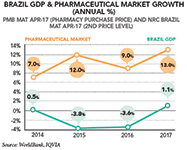
"In 2017, Brazil has exited a particularly challenging two-year recession period, where our country's GDP contracted by 3.8 percent in 2015 and by 3.6 percent in 2016," explains Cleiton Marques, CEO of the domestic powerhouse Biolab and president of the syndicate of the pharmaceutical industry in the State of Sao Paulo (Sindusfarma), which represents around 95 percent of all pharmaceutical marketing in the country and gathers together over 312 associate members overall.

Ricardo Barros, minister of health of Brazil
In December 2017, Brazil's Finance Minister Henrique Meirelles indeed announced that the Brazilian economy was expected to grow 1.1 percent in 2017 and 3 percent in 2018. "The country's recent exit from a two-year period of negative growth despite a troubled political context truly showcases how dissociated economics and politics are from each other in Brazil. In this regard, the country's economy has undoubtedly benefited from the quality of the policies implemented by Finance Minister Henrique Meirelles and from the active role played by the country's Central Bank, while some of the reforms and regulatory updates recently implemented have already started to bear fruit," explains Fraser Hall, country president of AstraZeneca in Brazil.

Jarbas Barbosa da Silva, director-president, Brazilian Health Regulatory Agency (ANVISA)
"In fact, in the wake of the crisis, all industry sectors constricted but two, one of them being the pharmaceutical industry. In 2015, at the height of the economic crisis, the pharmaceutical market was actually the fastest growing industrial sector of the Brazilian economy, growing 8.3 percent while all other sectors were largely in decline," reveals Leandro Safatle, executive secretary of the Brazilian Drug Market Regulation Chamber (CMED), which defines ceiling prices, benchmarks for wholesale and retail commercialization, and the minimum mandatory discount for purchases in the public healthcare system. "Our country has indeed been through particularly agitated times over the past few years, but the pharmaceutical industry in Brazil has always accommodated such crises," adds Nelson Mussolini, executive president of Sindusfarma.

Leandro Pinheiro Safatle, executive secretary, Brazilian Drug Market Regulation Chamber (CMED)
The sustained growth of the Brazilian pharmaceutical market has been nurtured by many different factors, which notably include particularly rapid demographic and epidemiological transitions. "I believe that some external observers still do not fully realize that Brazil is no longer a young country, as its age structure is actually pretty similar to those of European markets," explains Gaetano Crupi, president and general manager of Bristol-Myers Squibb Brazil, while it is expected that more than 30 million Brazilians will be over 65 year old by 2025.

Marco Fireman, vice minister and chairman, CONITEC
"Furthermore, healthcare stands as a constitutional right in Brazil since the promulgation of the 1988 Constitution, and this specificity has kept our market fully active even during the economic crisis, although we moved from a double digit to a single digit annual market growth" stresses Sindusfarma's Mussolini.

Nevertheless, one should not overlook that the outstanding resilience displayed by Brazil's pharmaceutical industry over the past few years is directly linked with one of the main shortcomings that still characterizes Brazil's public health system: a limited access to pharmaceutical products. "In a country where medicines purchasing comes as out of pocket expenses for around 76 percent of the Brazilian population, the recent economic crisis has had no impact on the growth of private pharmaceuticals sales, because patients cannot live without the medicines they need," adds Antonio Britto, the executive president of Interfarma, the main association gathering together local and international innovators in Brazil.
...BUT NOT A CASE OF BUSINESS AS USUAL.
Nevertheless, a subtler and more challenging reality actually lies behind the unstoppable momentum of the Brazilian pharmaceutical market. "The latter is undergoing rapid and deep changes, and we need to continuously adapt our business approach to the long-lasting consequences of the recent crisis, while seizing new growth opportunities triggered by the long-awaited economic recovery materializing," explains Alexandre França, CEO of Aspen Pharma Brazil, which in 2017 proudly became the best performing affiliate of the Aspen group in spite of the country's profound economic and political turmoil.

Nelson Mussolini, executive president, Sindusfarma
"When refining our analysis, we indeed see that the recent crisis has affected the retail market growth in terms of units, and the annual volume growth of this market decreased from 8.9 percent in 2015 to only 3.2 percent growth in 2017 (MAT April 2017)," documents IQVIA's Nilton Paletta. "Furthermore, high inflation in the 2015–16 period contributed to price growth, but-moving forward-inflation under control at the 3–4 percent level will entail that volume and price growth will be more balanced," he analyses.

Nilton Paletta, president Latin America, IQVIA
According to Sanofi's Pius Hornstein, a new norm is underway. "In this context, companies operating in the retail segment will need to follow a demand strategy, which implies a heightened focus on the quality of their treatments, the quality of their indications, the quality of their patient population segmentation, and so on. As the economy should slowly recover and Brazilians' purchasing power increases, it is expected that this will foster further demand in the out of pocket segment. Overall, this situation is by no means 'Alice in Wonderland,' but we are seeing a new positive normality, and we don't expect a return to the difficulties of the past few years," he predicts.

While the latest data from IQVIA indicate that the retail market will increase by 7–8 percent in 2018, the latter also forecasts that its mid-term growth should revolve around a CAGR of 7–8 percent between 2018 and 2021 in nominal terms. "While this is lower than the two digit growth we saw in the 2000s, it is still very attractive compared to the global average of two to three percent," highlights Hornstein.

Pius S. Hornstein, country chair and general manager pharma, Sanofi
In the meantime, the Brazilian non-retail market has been growing 11.6 percent (PPP and 2nd level-MAT April 2017) according to IQVIA, through the strengthening of a rather complex set of dynamics. In the public sector, which made up around 57 percent of the non-retail sector, the growth has been mainly driven by new product incorporations and increasing expenditures in high complexity diseases (mostly hepatitis C, HIV, immunology, and oncology).

Antonio Britto, executive president, Interfarma
"However, the institutional channel in Brazil remains highly volatile and it is hard to predict if the current growth will be sustainable moving forward. So far, the Ministry of Health's budget for medicines has increased 12 percent in 2016 and 10 percent in 2017, but a recent legislation has capped public spending's increasing to inflation rate for the next twenty years. In the meantime, we see that the frequency of incorporation of innovative products into the Brazilian public health system (SUS) has been improving over the past few years, although it is still suboptimal," reasons Paletta.

Cleiton Marques, CEO, Biolab and president, Sindusfarma
In this regard, the working of Brazil's institutional system has so far diminished the negative impact of the economic crisis, as the government could not cancel ongoing multiple-year tenders. "As a result, the Ministry of Health has been mainly focused on reaching a greater level of efficiency across the system and differing the payment of medicine-related bills-but no tremendous changes have yet occurred," stresses Interfarma's Britto, before adding that "we nevertheless cannot overlook the fact that the past economic crisis has tremendously decreased budget availability, which naturally casts a shadow on public market's growth prospects."

Rolf Hoenger, president and general manager, Roche Pharma Brazil
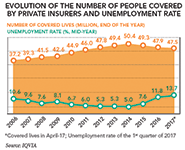
With 25 percent of its 207 million population covered by private health plans, Brazil holds the second largest private health insurance market by population in the world. "In Brazil, the vast majority of health plans are however contracted by employers, which means that the share of the Brazilian population having access to the private health market is directly correlated to the evolution of the unemployment rate. Over the past three years, this aspect has gained a tremendous importance in the eyes of pharmaceutical executives, as the unemployment rate grew from 5 percent to 13.7 percent between 2014 and 2016, and almost three million people lost access to the private health market," adds Roche's Hoenger.

Fraser Hall, country president, AstraZeneca
"Given that unemployment usually impacts entire households, we can moreover extrapolate that at least 45 million Brazilians do not hold the financial means to access products that are only available through the private healthcare system," further highlights Haig Yeghiaian, country manager of LEO Pharma. "Taking into consideration both public and private channels, we expect that Brazil's non-retail market will increase by 5–6 percent in 2018," explains Paletta.
LOOKING BACK WITH PRIDE
In parallel to market growth, the Brazilian ecosystem has clearly distinguished itself over the past years through the implementation of strategic regulations and public programs that showcase the country's ambitions to tirelessly upgrade its regulatory and healthcare frameworks. "Brazil is truly driving healthcare innovation across Latin America, while many countries in the region (and overseas) could inspire themselves from the policies implemented in Brazil over the past twenty years," highlights José Almeida Bastos, president Latin America at Merck & Co., "and the progress made by Brazil is truly impressive and praiseworthy, while the latter truly comes as a result of an enduring commitment to healthcare displayed by successive governments."

Norberto Prestes, director, ABIQUIFI
A first aspect that perfectly illustrates Brazil's recent achievements relates to the country's regulatory framework, with ANVISA holding the double mission of ensuring that Brazil meets the highest standards globally while integrating the specificities that come with the Brazilian market's rapid growth and its ongoing consolidation. "In this regard, we have conducted a comprehensive review of our agency's process, having the twofold objective of strengthening our registration processes and making Brazil a more transparent and predictable ecosystem for all stakeholders," explains ANVISA's Barbosa. Although it was only created in 1999, "the agency has made huge progress on the international stage too, as ANVISA joined the International Conference on Harmonization (ICH) in November 2016 alongside the US FDA, the EU EMA, and Japan's PMDA, which stands as an additional evidence of the high level of expertise developed by Brazil's regulator," highlights Bruno Costa Gabriel, managing director of Janssen Brazil. "Being accepted as a regulatory member of the ICH stands as a great recognition of the level of maturity that ANVISA has already been able to reach and highlights the quality of our regulatory processes, while providing us with a heightened influence in the global discussion for regulatory harmonization," continues its director-president Jarbas Barbosa. "Furthermore, we expect that ANVISA's membership to ICH will broaden the export prospects of Brazilian manufacturers," stresses Norberto Prestes, director of the Brazilian Pharmochemicals Manufacturers Association (ABIQUIFI).

Alexandre França, CEO, Aspen Pharma Brazil
On the healthcare side, the Ministry of Health (MoH) has recently implemented a genuine paradigm shift in moving from a top-down approach-where the MoH used to be the sole entity in charge of policy design while the States and the Municipalities were only entitled to these policies' implementation-to a new model based on joint-resolutions including all parties. "This collaborative approach is aimed at enabling a higher level of commitment across all layers of our health system and its four million workers, especially through a more efficient management of the SUS' BRL 250 billion [USD 81 billion] annual budget," explains Minister Barros, "while one should not forget the remarkable comprehensiveness of Brazil's public health system, which provides all Brazilian citizens with access to more than 4,500 medical procedures and 860 pharmaceutical products," he adds.

Furthermore, Brazil can boast of "being the only country in Latin America to strictly base the selection of new technologies entering the public health system on scientific evidence," according to Marco Fireman, vice-minister and chairman of the National Commission for the Incorporation of Technologies (CONITEC), created by law in April 2011. Since its set up until April 2017, 121 medicines were incorporated by CONITEC into the Brazilian public health system (SUS), "including-over the past months-treatments for widely prevalent diseases around the country such as hepatitis C, as well as technologies increasing adherence for Alzheimer's disease and products indicated for rare diseases," highlights Fireman.
"Brazil can truly pride itself on the excellent level of coverage today available for HIV, hemophilia or hepatitis C products through the SUS," confirms Roche's Rolf Hoenger, while "Brazil's National Immunization Programs (NIP) has now become a global reference in terms of access and coverage," adds José Almeida Bastos, Merck & Co.'s president for Latin America. "One of the great things about Brazil is that once they decide to do something, they do it very boldly and sustainably. Vaccinations are one of the biggest government-sponsored programs, and the government is also building an excellent HIV treatment program, leading the WHO to recently recognize Brazil as a global example in this disease's management," highlights Alexei Kolchin, general manager of GSK Brazil. "HIV is an area where we have some of the most advanced molecules on the market, and Brazil has been recently incorporating some of our innovative products into the public system's reimbursement lists, which stands as fantastic news for Brazilian HIV patients," confirms Cesar Rengifo, senior vice president & area director Emerging Markets West at GSK.
Although these eye-catching achievements must be praised, recent political turmoil and economic turbulence has seemingly delayed the implementation of new, highly needed bold moves that would bring Brazil's healthcare ecosystem to the next level. "This aspect is particularly crucial as Brazil truly stands as a country that requires a clear 25-year vision to further move forward. Day-by-day management will not allow us to face the huge challenges posed by our rapidly aging population, the evolution of our country's epidemiological profile, and the increasing prevalence of cancer or rare diseases, at the moment we definitely need to collectively look at the way the private and public sectors interact," warns Interfarma's Antonio Britto.
Life sciences companies in Latin America www.cbinet.com/pcclatinamerica are stepping up to combat bribery and corruption challenges. Attend CBI’s PCC Latin America to learn how to strengthen compliance programs, increase transparency and commit to a culture of ethics.
MAPPING OUT NEW CONQUESTS
Looking forward, room for improvement lies in both the regulatory and access sides. From a regulatory standpoint, the Brazilian context might seem quite disconcerting for a foreigner, as Brazil has successfully solved crucial issues with regards to legal predictability, the respect of intellectual property, and the strength of the country's pharmaceutical market, but it still lags behind concerning several more minor issues, such as registration timelines and red tape.

Alexei Kolchin, general manager, GSK
"At the end of the day, ANVISA's processes and requirements are closely aligned with those of the FDA in the US or NICE in the UK. What sets Brazil apart from these markets is its unpredictability, and this also applies to registration timelines and the pricing approach by regulatory authorities," explains Celgene's Luciano Finardi. "Speed to market in Brazil is still very slow, whereas we should be surfing the same wave as Europe and the United States. When international companies take into account this unpredictability as they calibrate their product launches, they sometimes favor a wait-and-see approach, observing competitors make the first move," he continues. Leveraging ANVISA's recent admission to ICH, one can however expect Brazil to be evermore aligned with the world's most advanced markets in the coming years.

Luciano Finardi, country manager, Celgene
"In the context of our recent ICH membership, we will work on addressing two of the main challenges that lie ahead for national regulatory bodies: first, ensuring a greater similarity between the frameworks of different pharmaceutical markets around the world, and, second, building up mutual trust among regulatory authorities in order to favor multiple country processes and authorizations-rather than individual, national requirements," explains ANVISA's Jarbas Barbosa. "In this regard, we recently saw that the US FDA and the EMA jointly assessed the market approval of a new pharmaceutical product-these kind of eye catching initiatives clearly needs to be replicated and expanded in the future," he highlights.

Nevertheless, "one of the greatest challenges at stake for Brazil's ecosystem is to square two competing dynamics: on the one hand, a rising healthcare demand driven by Brazil's rapidly aging population, and-on the other-the integration of new technologies that make health costs increase much faster than the inflation rate," states Minister Barros. "In Brazil, most government and regulatory officials are particularly keen on highlighting that Brazil's SUS stands as the largest government funded universal health system in the world; however, limited budget availability and the exceptional size of the population covered also implies that only basic products are integrated into the SUS. To give you an example, a cancer patient who exclusively relies on the public healthcare system would most likely only be treated with first-generation therapies," explains Celgene's Finardi. "Nevertheless, we
cannot blame CONITEC for this situation; every new innovative treatment reaching the Brazilian market puts the commission in an untenable situation, where allocating resources to include innovative treatments is extremely difficult as our country remains marked by the persisting significance of more basic health issues, such as infectious or cardiovascular diseases," he adds.

Fernando Itzaina, president and CEO, FQM Group
Resource allocation at the SUS level has become even more critical with the disruption caused by the rapidly growing judicialization of health, which relates to Brazilian patients filing lawsuits to access a given healthcare product or service through the SUS, leveraging the fact that healthcare in Brazil is a constitutional right. "In 2016, the federal government, the state and the municipalities all together spent more than BRL 7 billion [USD 2.2 billion] as part of health-related lawsuits, which is particularly substantial," explains Minister of Health Barros. "In this regard, the recently implemented S-code system will provide us with a better control of all legal expenses involving the provision of healthcare and allow us to crosscheck information in order to combat fraud," stresses the Minister, " while we also set up expert centers in partnership with the National Council of Justice to help judges for health-related lawsuits," he adds.

Bruno Costa Gabriel, managing director, Janssen
In the meantime, CMED's Leandro Safatle is looking forward to establishing a set of rules that will prevent companies from unlawfully taking advantage of judicialization of health, as the latter will receive a penalty equivalent to that addressed to companies selling their product above the regulated, authorized price. In addition to better controlling the excess and fraud related to the judicialization of health, Brazil's Ministry of Health also recently implemented a stringent control and review of the SUS' spending. "As per medicines, comprehensively reviewing all purchasing schemes allowed us to save over BRL 4 billion [USD 1.3 billion] between 2015 and 2016-out of a total budget of BRL 18 billion [USD 5.77 billion] annually allocated to medicine purchasing," stresses Minister Barros.

Mauro Loch, president and general manager, Amgen
However, when it comes to ensuring the long-term financial stability of the largest public health system in the world, more radical steps might be needed in the near future. "I believe that the private system will gain in importance in the upcoming years and we will see the implementation of a co-payment system, just like it happens in some European countries like Italy and Spain, as it is a good way to regulate the system: once the individual is helping with the payment of treatments, misuses tend to be tremendously reduced," believes Sindusfarma's Mussolini.

Samuel Mauricio, vice-president, Octapharma
While the recent economic crisis has led Brazil's MoH to take a (relatively unprecedented) stance on public spending, pharmaceutical executives in Brazil have unanimously highlighted the urgent necessity of further bridging the huge access gap that exists between the 160 million Brazilians that exclusively rely on the public system and the 47 million that have access to the private healthcare market-especially in some well-identified therapeutic areas. "Brazil stands as one of the Latin American countries where addressing the oncology conundrum is truly a public health necessity, and half-hearted measures will not bring satisfactory outcomes to patients exclusively dependent on the SUS-in a nutshell, Brazil must completely overhaul its oncology model at both the treatment pathway and access levels," stresses Merck & Co's president for Latin America, José Bastos.
While Brazil is a market that still displays huge social needs, there is a strong consensus among the entire ecosystem on the necessity to improve access to innovation, "but this responsibility should not only weigh on the government's shoulders," highlights Fernando Itzaina, president and CEO of FQM Group (FQM), a company that is part of the Argentina-based Roemmers Group, which gathers together multiple pharmaceutical and healthcare companies across Latin America. "When it comes to innovation access in Brazil, we are at the dawn of a new era-this is not an assumption, this is a necessity," states Interfarma's Britto.
Life sciences companies in Latin America www.cbinet.com/pcclatinamerica are stepping up to combat bribery and corruption challenges. Attend CBI’s PCC Latin America to learn how to strengthen compliance programs, increase transparency and commit to a culture of ethics.
TRANSFORMATIVE AMBITIONS
"Although osteoporosis, hematology, and oncology have their own spaces in Brazil's private market, we do not aim to become a private market focused company, and we will remain committed to and continue exploring both segments despite the spending freeze recently decided by the Brazilian government and its negative impact on the budget of the Ministry of Health," states Mauro Loch, general manager of Amgen Brazil, thereby illustrating the efforts of many international innovators to find innovative ways to make their treatments accessible through the SUS.

"Pharmaceutical companies have a crucial role to play in building a more sustainable health system in Brazil, especially through the establishment of Partnerships for Productive Development (PDP)," highlights Minister Barros. These PDPs notably encompass technology transfers and the local manufacturing of patented, in-need products through partnerships with Brazil's public laboratories, therefore significantly lowering prices and extending healthcare access to all Brazilians.

Heraldo Marchezini, CEO, BIOMM
"As we speak, scheduled investments related to PDPs amount to over BRL 6.5 billion [USD 2.1 billion] and will create more than 7,500 new jobs-only for the set-up of manufacturing facilities that will handle these technology transfers. In this regard, we invite new pharmaceutical companies owning strategic products and patents to reach out to the Ministry of Health and set up or develop their footprints in Brazil through PDPs," continues Minister Barros.
As the government identified more than 180 strategic technology transfers, PDPs have become one of the hottest topics among Brazil's pharmaceutical industry, and some innovators have already successfully positioned themselves in this segment. "So far, only one company has actually successfully engaged in a PDP, transferred the technology to the local partner, where the latter has subsequently successfully produced, distributed and sold the resulting products in Brazil. That company is Sanofi, through Sanofi Pasteur, with our flu vaccine PDP. This gives you an indication of not only our commitment to Brazil but our capabilities," highlights Pius Hornstein, Sanofi Brazil's country chair. "Earlier this year, the Ministry of Health moreover issued a new PDP list with over 50 priority products, including three core products for Sanofi. After a long internal discussion at both corporate and local levels, we decided to submit a PDP proposal for each of them. This is highly notable as it is not easy for a MNC to engage in such a program, especially for core products. There is still a long path to go as the Ministry of Health needs to do a first selection, then an evaluation, and then the negotiations, but it was a significant decision for Sanofi in Brazil," he adds.
In the same way, Swiss-based Octapharma, one of the largest human protein manufacturers in the world, is ready to embrace the same transformative commitment to Brazil after two PDPs for the supply and technology transfer of recombinant Factor VIII failed to deliver on their premises. "We decided to seize this exciting opportunity and submitted in June 2017 a new, holistic project, whose fundamental approach is to develop a more integrated and complete partnership model, which would allow the government to fully maximize the investment conducted by our company," explains Samuel Mauricio, vice-president for Brazil at Octapharma.
"Furthermore, we truly looked at leveraging Octapharma's technical leadership to develop a tailor-made solution adapted to the specific needs of the Brazilian ecosystem, including the risks posed by the high prevalence of infectious and tropical diseases in the country. As a result, we integrated in our project a proprietary technology that enables the virus inactivation of the plasma for all relevant viruses from a transfusion point of view-including the Zika, Dengue and Chikungunya virus-which will provide Brazilian clinics and hospitals with a heightened transfusion safety," he continues.
"In the grand scheme of things, this PDP would truly allow Brazil to move up to a new dimension, as only a very limited number of countries in the world have managed to become independent for the production of plasma proteins and recombinant factor VIII. In the case of Brazil, this aspect is even more crucial as the country holds the third largest hemophilic population globally," concludes Samuel Mauricio, vice-president for Brazil at Octapharma, to illustrate the game-changing potential of PDPs in critical therapeutic areas.
Although 104 out of the 180 PDPs prioritized by the MoH have already been agreed on, only three of them (according to Interfarma data) have actually completed the entire technology transfer process, leading some stakeholders to question these PDPs' design and execution framework. "Interfarma's position is that the government should concentrate its efforts on the most innovative technologies, i.e. the ones for which there is no free market setting. Second, we need to develop a model where Brazilian laboratories are actually able to receive all technology transfers agreed, and-finally-we must ensure PDPs are developed in legal and ethical ways that would bring real technological improvements to Brazil and/or decrease product prices in comparison to a free market situation," summarizes Interfarma's Britto.
Nevertheless, PDPs are far from being the only way forward for pharmaceutical companies aiming to have a transformative impact on the SUS' access paradigm-even in some of the most underserved areas such as oncology. "At Roche, the opportunities that we have been able to seize stem from the structural specificities of the Brazilian market: a federal republic comprising 26 states plus the district capital Brasilia, and over 5,600 cities-and this structure is reflected across the Brazilian model of healthcare," stresses Rolf Hoenger, president and general manager of Roche Pharma Brazil. "In 2014, we therefore set up market access teams entirely dedicated to identifying access opportunities at the state level for products that haven't yet been included into CONITEC's federal reimbursement list. When narrowing the access equation to the state level, we were able to refine our access strategy according to these states' specific healthcare needs. For example, the prevalence of cervical cancer is relatively high in some northern states of Brazil, where these states' secretaries of health would more likely consider cervical cancer as a health priority-and therefore prioritize resource allocation for the reimbursement of innovative treatments in this area," he adds, while some of these state-based access models were actually designed through co-creation workshops gathering together local secretaries of health and animated by Roche employees, as part of the company's Inovamentes program.
"In the meantime, we have conducted a comprehensive mapping of the access scenario for breast cancer, lymphoma, and colon cancer in 148 different cities, including the level of disease awareness and the strength of the primary care and diagnostic capacities. Out of these 148 Brazilian cities, we have already been able to implement tailored and impactful access solutions in more than 120," he concludes.
Life sciences companies in Latin America www.cbinet.com/pcclatinamerica are stepping up to combat bribery and corruption challenges. Attend CBI’s PCC Latin America to learn how to strengthen compliance programs, increase transparency and commit to a culture of ethics.
THE HMO EQUATION
Given that private health plans covered almost 48 million Brazilians in 2017, pharmaceutical executives also identify great opportunities to increase innovation access by more closely partnering with HMOs. "At Merck KGaA, we have already managed to successfully include several products in the formularies of private health insurances, but we definitely have room for further improvement in this area," highlights Guilherme Maradei, managing director Brazil and general manager biopharma at Merck KGaA.

"For mental health products, there are only a few HMOs that are actually open to the idea, as including these products in their reimbursement lists would come at a very high cost for these companies," adds Josiel Florenzano, managing director for Lundbeck Brazil and South Cone. "As per Lundbeck, we have not yet engaged in this process, but I believe it truly embodies the way forward for our country, and I expect that more and more companies' representatives will be focus on private health insurers within the next ten years, especially as the government recently announced that public spending would be capped to inflation rate for the next twenty years, therefore limiting opportunities to increase access in the public sector," he stresses.

Haig Yeghiaian, country manager, LEO Pharma
Talking about HMOs and private payers, the sector has moreover been consolidating over the past five years, and the total number of private payers has significantly decreased from 973 to 790 between 2012 and 2017. "In the meantime, the share of HMOs covering more than 250,000 lives has increased from 50 to 55 percent and the trend is set to continue in the coming years," highlights Nilton Paletta, president Latin America at IQVIA.

Alex Carvalho, general manager, Pierre Fabre
Nevertheless, as the vast majority of health plans are contracted by employers, the latter are typically looking for the best deals possible and therefore regularly switch contractors. "The average contract length between a company and a HMO in Brazil is around three years, which nurtures two negative dynamics: first, the patient is somewhat disenfranchised from the relationship with his healthcare providers, which generates wasteful spending," explains AstraZeneca's Fraser Hall. "Second, HMOs struggle to track long-term patient records and outcomes, which does not contribute to driving costs down either. When combining the impact of these two dynamics with the fact that healthcare cost in Brazil has been increasing on average by 18 percent a year, it is easy to understand why HMOs are pushing back against the inclusion of new, innovative treatments. We however identify a group of "early adopter" HMOs covering around 15 percent of the 48 millions Brazilians using the private market, which are more open to reimbursing new treatments and use this specificity as a key differentiator vis-à-vis their competitors," he adds.

Josiel Florenzano, managing director, Lundbeck Brazil and South Cone
"In this context, broadening access in the private sector will rely on our capacity to develop innovative schemes with HMOs-and Pierre Fabre Brazil aims to be an active contributor to this approach. Although the private insurance market is still extremely fragmented in Brazil, we can start working with some of the country's largest institutions and jointly design access models, which could then be used as blueprints by other HMOs down the road," explains Alex Carvalho, general manager of Pierre Fabre Brazil.
Nevertheless, in Brazil, private health plans do not cover treatments for chronic diseases and essentially focus on in-patient care, which poses another challenge for companies holding these products in their portfolios. "So far, 100 percent of the sales of our evolocumab for high LDL cholesterol come as out-of-pocket spending, as it is not accessible through Brazil's public health system either. As a result, one of the main priorities of our access teams is to closely work with private insurers to improve the coverage of this product in the private healthcare market," explains Mauro Loch, general manager of Amgen Brazil.
"Fulfilling this objective will however require completing a true paradigm shift that would see private insurers' cost assessment approach evolving from a product- to a disease-centered model, which entails providing our partners with detailed insights into the economic burden of the disease, the average costs associated with emergency events, as well as the savings that could be made thanks to the clinical outcomes offered by our product," he stresses.
While patient-centricity is high on the agenda of most pharmaceutical companies in Brazil, the latter should not overlook the importance of being payer-centric either. "It is extremely difficult for Brazilian payers to assess products based on pharmacoeconomic studies performed in European or North American health systems-whose cost structure is completely different from that of the Brazilian healthcare system. Nevertheless, we see that most companies in Brazil are still reluctant to perform local pharmacoeconomic studies," points out Celgene's Luciano Finardi.
Furthermore, Brazil's private payers seem to still lag behind the US in terms of label enforcement, leading companies to furnish heightened efforts to see approved, life-changing products being included in HMOs' formularies. "Looking at BMS' PD-1 immune checkpoint inhibitor nivolumab which has already been approved in five indications in Brazil, our country's HMOs do not automatically add this ground-breaking product into their guidelines. In contrast to the US, we therefore have to negotiate on an individual basis with HMOs and showcase this product's benefits to ensure they really allow physicians to prescribe nivolumab and reimburse it to their beneficiaries," reveals Gaetano Crupi, president and general manager of BMS in Brazil.
Finally, another identified room for improvement concerns targeted therapies-a model that has not yet been embraced by both the government and private insurances. "As a result, the pharmaceutical industry in Brazil has so far been financing most of the diagnostic costs in Brazil, which stands as another challenge to address when further strengthening our relationship with HMOs," concludes AstraZeneca's Fraser Hall.
Life sciences companies in Latin America www.cbinet.com/pcclatinamerica are stepping up to combat bribery and corruption challenges. Attend CBI’s PCC Latin America to learn how to strengthen compliance programs, increase transparency and commit to a culture of ethics.
FINE-TUNING COMMERCIAL STRATEGIES
In parallel to transformative approaches aiming to shift the country's access paradigm in both the private and public healthcare markets, companies cannot afford to lose sight of their commercial performance either-especially given that Brazil's sheer market size nurtures extremely high expectations from headquarters. "Being successful in Brazil and meeting local stakeholders' and patients' needs truly requires building a country-specific portfolio, which might differ from those that companies have in European and Asian markets," warns Haig Yeghiaian, country manager at LEO Pharma.

"Furthermore, from a commercial standpoint, Brazil cannot be approached as "one country," as there are strong cultural, social, demographic, and climatic differences between all the states," explains Fernando Itzaina, president and CEO of FQM Group (FQM). "This diversity has an impact on the pharmaceutical market, as epidemiological and demographic profiles as well as healthcare needs vary significantly from one state to another, which in turn requires pharmaceutical companies to adapt their strategies accordingly. To make the most of the market's huge potential, companies and their management teams must also cover strategic hubs scattered across this huge country, including the political capital Brasilia, the country's economic center Sao Paulo and other large cities such as Rio de Janeiro, Salvador, Fortaleza, and Belo Horizonte" he adds.

Guilherme Maradei, managing director, Merck KGaA
"In this regard, it is absolutely crucial to ensure our treatments are truly available to patients, which actually goes beyond affordability: as a company, we hold the mission of guaranteeing to our partners and patients that our life-changing products are easily accessible to all patients across Brazil's huge territory," explains LEO Pharma's Yeghiaian.

Fulfilling this endeavor appears evermore challenging when considering that some parts of the Brazilian pharmaceutical value chain have not matured at the same pace as in other Latin American countries. "Mexico, for instance, is much more advanced in aspects like distribution and pharmacy chains. In particular there is no very dominant pharmacy chain present at country level in Brazil, and I expect to see a significant transformation within the Brazilian healthcare landscape in the next five to ten years," highlights Sanofi's Pius Hornstein.
Although the Brazilian value chain is still under consolidation, one single distributor today holds a market share of over 40 percent, which has negatively impacted the risk exposure of some affiliates. "We are now engaged in the ongoing process of designing a new sales structure for Aspen Brazil, which includes further strengthening our traded marketing division to display a heightened focus on pharmacy chains and distributors," confirms Aspen Pharma Brazil's CEO, Alexandre França.
Furthermore, Brazil's pharmaceutical value chain is still characterized by resistance to change, as recently experienced by Aspen Pharma, which established itself as one the fastest growing pharmaceutical companies in Brazil in 2017 through radical decisions taken at both internal and external levels. "Bolstering structural changes was clearly more difficult at the external level than on the organization side, as most managers across the value chain still seem to prefer accommodating themselves to the sector's ups and downs rather than favoring or welcoming disruptive approaches," explains Aspen Pharma's França. "We however decided to question this status quo-at the end of the day, why should we wait for the market to recover, while bold strategy changes could enable us to outperforming the market even during crisis times? Every problem is an opportunity in disguise. While our country was facing its worst economic crisis in many decades, some market niches have still been growing at a rapid pace," he continues.
For innovative products, "it is however much more difficult to reach the same level of market penetration than 15 years ago," stresses Lundbeck's Florenziano: "while the overall size of the Brazilian antidepressant market has soared over the past decade and has now overcome the USD one billion mark, the number of generics competitors has increased accordingly," and the latter have been driving most of the retail market's volume growth since the beginning of the recent economic crisis. Fortunately, innovators can still rely on the strong performance of their mature brands to propel the affiliates' long-term growth. "In the Brazilian CNS market, the brand erosion usually hovers around 15 percent (in market share) in the first year that an innovative product goes off patent, while-in Europe or in the US-it may however reach up to 85 percent in the first year of patent loss," adds Lundbeck's Florenziano.
Another commercial opportunity to seize relates to the fact that-in Brazil-prescribers still hold a real decision power even after generic entry, which is not the case anymore in European countries, where prescribers have to first and foremost comply with payers' reimbursement lists. "In Brazil like in many other Latin American markets, we do see prescriptions changes happening at the point of sales; however, physicians' high influence entails great development opportunities for companies that have managed to build a great reputation for themselves," explains Pierre Fabre's Alex Carvalho.
In this context, fully leveraging one company's leadership in a given therapeutic area and among prescribers also emerges as an optimal way to unlock Brazil's market potential and outperform competition. "In order to further accelerate the growth of our oncology portfolio, we set up in 2015 commercial partnership with EMS, the largest domestic company in Brazil and one of the most significant pharmaceutical companies in all Latin America," continues Carvalho. "As part of this partnership, we are taking care of the marketing and promotion of a very important leukemia product, which is manufactured by EMS before complying with a second quality control at Pierre Fabre's Brazilian distribution center. Our partnership with EMS actually emerges as the starting point of our licensing strategy, and we are currently working on forging other agreements with international companies in the oncology field, which I cannot disclose at the moment," he adds.
Moving forward, distributors and pharmacy chains are not the only part of the Brazilian value chain that may experience significant consolidation, and Brazil's pharmaceutical companies could renew the high level of M&A deals that transformed the industry until 2013-before significantly decreasing when the crisis broke out. "Although it is extremely difficult to predict how the situation will evolve, moving from a double digit to a single digit market growth might prompt companies to consider gaining new market shares through acquisitions," explains IQVIA's Paletta.
In this regard, FQM Group (FQM), originally Farmoquímica, distinguished itself in 2017 with the acquisitions of both DIVCOM's pharmaceutical division in May 2017 and dermatology-focused Melora in January 2016, allowing the company's revenues to grow by 144 percent between 2014 and 2017, with 64 percent coming from inorganic developments (including the acquisitions of DIVCOM and Melora). "Increasing a company's revenues from USD 10 to 20 million is no easy task but it is far from being impossible in Brazil-even if it represents a 100 percent increase of the revenues. However, this objective becomes even tougher when these revenues reach a higher level-let's say over USD 300 million," explains Fernando Itzaina, President and CEO of FQM.
Life sciences companies in Latin America www.cbinet.com/pcclatinamerica are stepping up to combat bribery and corruption challenges. Attend CBI’s PCC Latin America to learn how to strengthen compliance programs, increase transparency and commit to a culture of ethics.
BREAKING DOWN BARRIERS
If mergers and acquisitions had the wind in their sails during the BRICS boom, it is partly due to fact that Brazil stands as a very complex and costly market to enter for many international companies. This specificity has not vanished over the past few years: Brazil is still characterized by high operational costs, one of the most complex tax systems in the world, and substantial logistical challenges inherent to the size of the world's fifth largest country. As a result, many international companies have not yet taken the leap of faith to enter the promising but perilous Brazilian market. "In this context, FQM's mission is to ensure that all these companies' products do not remain inaccessible to Brazilian patients because of market barriers, and we established in-licensing partnerships as one of our main development pillars," explains FQM's Itzaina. "We actually decided to go one step further with the set up of our own innovation center, which is mainly aimed to adapt foreign products to the specificities of Brazilian regulations: although ANVISA's requirements are more and more aligned with those of its most prestigious counterparts, regulatory differences still exist and require investments from our side to ensure foreign products can be swiftly available to Brazilian patients," he adds.
When it comes to bringing to Brazil some of the world's best products, Biomm-the first biotech ever set up in Brazil-recently stood out from the crowd through the inking of two eye-catching partnerships in 2017. "In the early summer of 2017, we entered into an agreement with the US company Mannkind for the marketing and distribution of a very innovative fast-acting, inhaled insulin, which will make Brazil the first country outside the US to receive this life-changing product," reveals Heraldo Marchezini, CEO of Biomm and one of the leading executives in the Brazilian biopharmaceutical sector. "The product was submitted to ANVISA at the end of October 2017 for product approval, and this filing actually includes new label information," he adds.
As a true biotech company, Biomm however does not plan to limit itself to the diabetes field, as proven by the company's recent partnership with South Korea's Celltrion Healthcare for a trastuzumab biosimilar prescribed for the treatment of breast tumors with HER2-Positive receptor status, which affects over 25 percent of invasive breast cancer globally. "As a company, we are particularly proud to have reached an agreement with Celltrion, which stands as the first biopharmaceutical company to have a biosimilar monoclonal antibody approved in Europe. Furthermore, this partnership perfectly embodies Biomm's commitment to explore a variety of critical therapeutic areas, while ensuring that Brazilian patients hold more affordable and accessible options to treat the disease," stresses Marchezini, before adding that "in the oncology area, we see very innovative treatments reaching the Brazilian market, but the latter will remain inaccessible to most patients if pharmaceutical companies do not make the effort to bring biosimilars in the meantime."
Life sciences companies in Latin America www.cbinet.com/pcclatinamerica are stepping up to combat bribery and corruption challenges. Attend CBI’s PCC Latin America to learn how to strengthen compliance programs, increase transparency and commit to a culture of ethics.
PREPARING FOR AN UNCERTAIN FUTURE
Beyond its sheer size or annual growth rates, the most exciting specificity of the Brazilian market in the eyes of pharmaceutical executives probably lies in the creativity and flexibility that it requires on both daily and strategic bases. "That's the beauty of Brazil: the market is big enough to be extremely innovative at the affiliate level, while its strategic importance makes it easier to receive the resources required to experiment with innovative processes and-in turn-increase your chances of success," explains Rolf Hoenger, president and general manager of Roche Pharma Brazil, whose affiliate holds an innovation specialist fully dedicated to fostering creativity and supporting Roche employees in their innovative projects. "However, in such a complex and somewhat unpredictable environment, being resilient and holding firm to your long-term plan is absolutely paramount," advises Janssen's Bruno Costa Gabriel, while Sanofi's Hornstein highlights that "Brazil is a unique and fairly complex country that will constantly surprise you. As a company, if you can embrace how Brazil works and add something further of value, you can be successful."
Furthermore, operating at the executive level in Brazil requires constantly monitoring external trends and being extremely agile to adapt a large-scale organization to ever-changing industry winds. "As a result, I always keep a close eye on our affiliate's numbers, the performance of the industry through various indicators as well as other market data, and I believe this approach works well to anticipate changes in the environment, define and implement actions that help ensure our business is best positioned for growth at any given scenario," adds Guilherme Maradei, managing director of Merck KGaA Brazil, which grew by over 20 percent in volume in 2017, compared to a market increase of just over four percent.
"I also consider that the most successful global executives of the future must have an in-depth understanding of high-growth, strategic emerging markets-just like experience in developed markets has been important in the last decades. As countries like Brazil have gained importance in multinationals' growth plans, holding on-the-ground experience in this country is a valuable asset when it comes to shaping the global strategy of a multinational company," adds Maradei. "Finally, my suggestion to all executives coming to Brazil is to find ways to make a lasting impact in the country: we are truly privileged to work in an industry that changes lives, and Brazilians truly welcome foreigners that are eager to improve their country's healthcare paradigm-so do not miss this great opportunity," recommends AstraZeneca's Fraser Hall.
While the Brazilian economy is expected to recover at a pace that would make most European countries recently affected by economic recession green with envy, the key parameter to watch moving forward will be the impact of the November 2018 general elections, which so far display a very unpredictable scenario. "In the grand scheme of things, I believe that the future growth of our country's pharmaceutical industry will also largely depend on Brazil's capacity to further attract foreign investments," forecasts Sindusfarma's Nelson Mussolini. "Overall, all the conditions have been met to allow Brazil to become the third or fourth largest pharmaceutical market in the world; I am confident that this will happen one day, and those companies that will have been able to build strong operations and a long-standing presence in Brazil will indisputably reap the rewards of their commitments," he concludes.

Country Report: United Arab Emirates
March 1st 2020With an estimated annual growth rate of 10%, the Middle East is now consistently outpacing traditionally sought-after “pharmerging” heavyweights such as China and Brazil. The United Arab Emirates is at the center of this newfound momentum-asserting its own credentials as a prospective destination for big-ticket foreign investment in healthcare.