Critical Thought
Pharmaceutical Executive
IN OUR APRIL 2006 ISSUE, we will explore the results of a reader study—a" pulse check" as we think of it—on the topics of promotional programs, meeting spend, and compliance, cosponsored by Maritz Travel and Pharmaceutical Executive. The study asked industry leaders about their perceptions of their companies' physician meetings in terms of audience, format, and budget, as well as their views on compliance regulations.
IN OUR APRIL 2006 ISSUE, we will explore the results of a reader study—a" pulse check" as we think of it—on the topics of promotional programs, meeting spend, and compliance, cosponsored by Maritz Travel and Pharmaceutical Executive. The study asked industry leaders about their perceptions of their companies' physician meetings in terms of audience, format, and budget, as well as their views on compliance regulations. A total of 61 readers involved in compliance monitoring or management of promotional program budgets within the pharmaceutical industry responded.
Because of the small sample and non-scientific nature of the sampling, the results may not be representative of all Pharm Exec readers. Findings are more qualitative than quantitative, but they help to tell a story even without providing significant statistics. They begin to provide a picture of what your fellow industry executives believe.
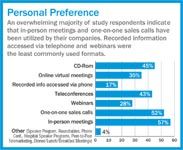
Some Highlights Include:
In-person meetings and one-on-one sales calls are the most commonly used meeting formats. Respondents reported that these meeting formats are most effective for conveying product information and gaining strong physician participation.
Many respondents feel that their company is doing only an average job of reaching the right audience and getting a good turnout for their promotional meetings, so there is still much room for improvement.
In general, study respondents feel that pharma companies have taken recent changes in compliance regulations very seriously.
• Two-thirds of respondents agree that the pharma industry is strictly following compliance regulations.
• Respondents report having dedicated compliance departments and legal departments to handle issues related to compliance requests.
On average, companies received 24 compliance requests during the past year; three out of five came from a state, and the rest from federal agencies.
Stay tuned for more info in the April 2006 issue of Pharm Exec.
Addressing Disparities in Psoriasis Trials: Takeda's Strategies for Inclusivity in Clinical Research
April 14th 2025LaShell Robinson, Head of Global Feasibility and Trial Equity at Takeda, speaks about the company's strategies to engage patients in underrepresented populations in its phase III psoriasis trials.
Beyond the Prescription: Pharma's Role in Digital Health Conversations
April 1st 2025Join us for an insightful conversation with Jennifer Harakal, Head of Regulatory Affairs at Canopy Life Sciences, as we unpack the evolving intersection of social media and healthcare decisions. Discover how pharmaceutical companies can navigate regulatory challenges while meaningfully engaging with consumers in digital spaces. Jennifer shares expert strategies for responsible marketing, working with influencers, and creating educational content that bridges the gap between patients and healthcare providers. A must-listen for pharma marketers looking to build trust and compliance in today's social media landscape.
Pfizer, GSK Gain ACIP Recommendations for RSV and Meningococcal Vaccines
April 18th 2025The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted to expand access to Pfizer’s respiratory syncytial virus vaccine Abrysvo for high-risk adults in their 50s and voted in favor of GSK’s meningococcal vaccine, Penmenvy, for streamlined adolescent protection.
