
Specialty drug sales, record-breaking M&A paired with tax synergies, and global expansion helped to bring a new face into this year’s Pharma 50 top 10, and substantially boosted the rankings of several others.

Specialty drug sales, record-breaking M&A paired with tax synergies, and global expansion helped to bring a new face into this year’s Pharma 50 top 10, and substantially boosted the rankings of several others.

Successfully commercializing a proprietary drug in China can be a Kafkaesque affair.

FDA officials willing to relocate to far-flung global cities should be congratulated for their herculean efforts to protect American patients. But funding cuts hamper the Agency's international presence.

Non-adherence, in the US alone, is a $100-billion-a-year problem. Healthcare players are touting patient education and engagement as the keys to better adherence rates. Ben Comer reports.

The Cancer Research Institute is clearing a path to the future of immunotherapeutics. Ben Comer speaks to its CEO and director of scientific affairs, Jill O'Donnell-Tormey

Pharm Exec’s Brand of the Year selections from the last few years are still alive and kicking, although some have aged more gracefully than others.
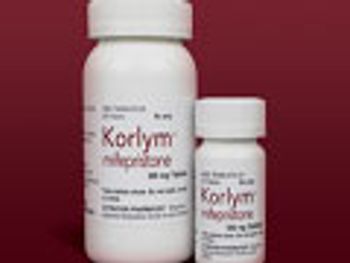
Pharm Exec's Brand of the Year recipients for 2014 are multiple sclerosis treatment Copaxone and KORLYM for diseases driven by excess production of the metabolic hormone, cortisol. We profile the journey of both drugs.

Ben Comer reports on how new pipeline therapies, public research investments, and a renewed sociopolitical focus are working toward a happier outcome for sickle cell disease patients.
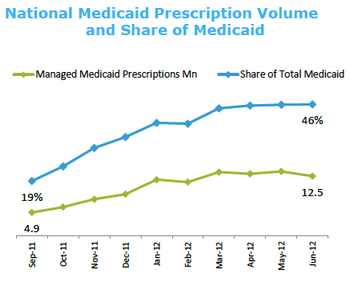
Cash-strapped states are shifting patients from traditional fee-for-service Medicaid coverage to managed Medicaid plans.

When the Generic Drug User Fee Act (GDUFA) was signed into law on July 7, 2012, its primary intent was to provide FDA with the additional resources necessary to expedite the review process for generic drugs, and combat the agency’s backlog of drug applications.

New products in development for women distressed by a lack of sexual desire and satisfying sexual experiences are causing a stir, but not for the reasons one might expect.

Pharma industry executives, FDA officials and Department of Justice attorneys speaking last week at CBI’s 11th annual Pharmaceutical Compliance Congress (PCC) offered solutions and threats to companies hoping to avoid enforcement actions.

Advocacy organizations and individual patients are getting more involved in every facet of the healthcare system, from drug R&D, to federal and state policy all fueled by the hour-to-hour passion of living with a disease.

For Big Pharma, the merits of strategic focus and operational discipline are more important than ever. It is time to be decisive - muddling through is so yesterday.

The IMS Institute for Healthcare Informatics worked up a methodology for assessing the effectiveness of pharma’s social media efforts across Facebook, Twitter and YouTube.

Presenters at the 32nd annual J.P. Morgan Healthcare Conference talked up value-based pricing, emerging market strategies, complex generics and new technology, from bedside devices and genetic sequencers to first-in-class mechanisms of action.
FDA’s new Breakthrough Therapies designation sped Pharmacyclics/J&J’s ibrutinib (brand name: Imbruvica) through regulatory review in four months, based on Phase 2 studies. Pricing and access issues, though, may not get resolved so quickly.
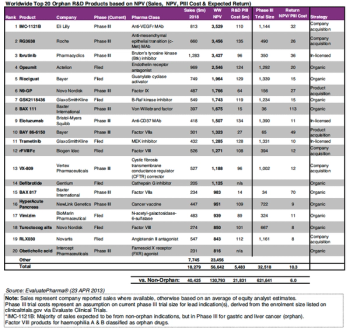
Orphan drugs were given short shrift in PharmExec’s 2014 Pipeline Report, but a couple of pipeline candidates targeting small populations did make the list, and are expected to earn big dollars in the next few years.

At the conclusion of the Cleveland Clinic's Medical Innovations Summit each year, 10 innovative technologies are unveiled before the audience, and designated as new and revolutionary tools for the treatment of disease and disability.

Predictive analysis has been used to great effect in the finance, tech, retail, and telecoms industries. Can it also improve decision-making and health outcomes in life sciences?

Planning to submit to the FDA today? Think again. The agency put a forced pause on key services and functions – and furloughed 6,620 FDA employees – as a result of last night’s government shutdown.

Post Exubera, inhaled insulin has been an uphill struggle with regulators.

Are electronic health records a viable channel for engagement? Ben Comer reports.

Novo Nordisk’s SVP of national diabetes sales speaks with PharmExec about a new field force technology initiative, implications of the Sunshine Act, and why Victoza sales won’t slow down anytime soon.

In the spirit of lawn chair cogitation and hammock-spun reverie, Pharm Exec invites you to submit your own verses-pharma-related, of course-to be published on our website in late August.

Scientific opinion has special protection under the First Amendment, but does that give pharma a free pass to BYOD – build your own dataset – publish a peer-reviewed article based on the data, and then promote it for competitive advantage?

Pharma and physicians worry about getting sunburned by the Sunshine Act.

Dr. Richard Gliklich, president of Quintiles Outcome and a professor at Harvard Medical School, highlights the most prominent post-approval issues and risks coming out of this year’s Post-Approval Summit, held on May 7th-8th.
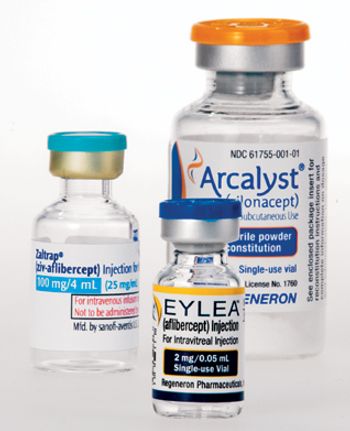
Led by two New Yorkers with a mindset that marries novel scientific insights to the processing power of novel technologies, Regeneron's success is a revolutionary challenge to the industry status quo.

Project Data Sphere aims to liberate clinical trial data sets from industry and academic vaults, in an attempt to catalyze cancer research and discovery.

Published: February 1st 2013 | Updated:

Published: March 1st 2013 | Updated:
Published: April 22nd 2014 | Updated:

Published: February 1st 2014 | Updated:

Published: November 1st 2013 | Updated:

Published: January 21st 2014 | Updated: