
Merck is actively enrolling patients across Phase III trials for four novel candidates for hematologic malignancies and solid tumors.

Merck is actively enrolling patients across Phase III trials for four novel candidates for hematologic malignancies and solid tumors.

Tina Fresta, BA, MPH is the Strategic Solutions Senior Manager at epocrates, keeps a close eye on the pharmaceutical and bio science industry shifts, leveraging those insights to inform both our partners and our company of the most strategic approach to reach business goals.

Starting April 1, 2024, Hyrimoz and an unbranded version of Humira manufactured by Sandoz will be covered across all CVS formularies.
The LillyDirect platform will allow patients who are prescribed the popular weight loss drug Zepbound to obtain the drug via Lilly’s at-home prescription delivery service.

Cretostimogene grenadenorepvec (CG0070) is currently being evaluated for the treatment of patients with high-risk Bacillus Calmette-Guérin–unresponsive non–muscle invasive bladder cancer with carcinoma in situ with or without Ta or T1 tumors.
Study results show an estimated 71.4% survival rate after both 24 and 36 months with aglatimagene besadenovec (CAN-2409) in combination with valacyclovir for the treatment of patients with pancreatic ductal adenocarcinoma compared with 16.7% in the control group.

Merger agreement includes the Gracell FasTCAR platform, which could significantly improve the efficacy of CAR T-cell therapies.

Filsuvez (birch triterpenes) is indicated to treat partial thickness wounds associated with junctional epidermolysis bullosa and dystrophic epidermolysis bullosa.
Merck’s Biologics License Application for V116, a novel 21-valent pneumococcal conjugate vaccine has been given a Prescription Drug User Fee Act (PDUFA) date of June 17, 2024.

Indication of Adbry (tralokinumab-ldrm) expanded to include patients 12 to 17 years of age with moderate-to-severe atopic dermatitis whose disease is not adequately controlled with topical prescription therapies or for whom those therapies are not advisable.

Pivotal trial findings show favorable clinical safety and efficacy data for mRNA-1345 in lowering the incidence of respiratory syncytial virus-associated lower respiratory tract disease.
Jemperli plus standard-of-care chemotherapy with carboplatin and paclitaxel, followed by Jemperli plus Zejula as maintenance therapy produced a statistically significant and clinically meaningful benefit in progression-free survival in patients with primary advanced or recurrent endometrial cancer.

Zoryve (roflumilast) topical foam, 0.3% is the first approved treatment for seborrheic dermatitis with a new mechanism of action in more than two decades.
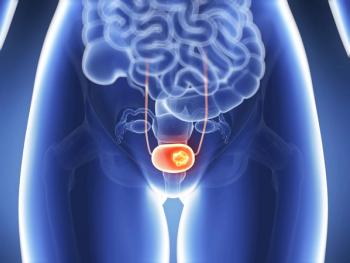
The FDA assigned a PDUFA date of April 5, 2024 for Opdivo (nivolumab) plus cisplatin-based chemotherapy for the first-line treatment of adults with unresectable or metastatic urothelial carcinoma.
The launch of the Elecsys HBeAg quant immunoassay adds to Roche's viral hepatitis testing portfolio.
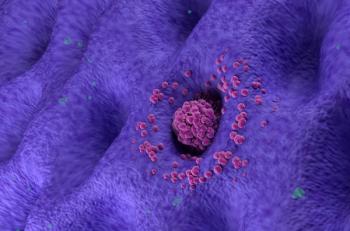
Merck's pembrolizumab (Keytruda) plus fluoropyrimidine- and platinum-containing chemotherapy approved for the first-line treatment of patients with locally advanced unresectable or metastatic, HER2-negative gastric/GEJ adenocarcinoma.

Repotrectinib (Augtyro) is a next-generation, potential best-in-class tyrosine kinase inhibitor approved to treat locally advanced or metastatic ROS1-positive non-small cell lung cancer.

The eye drops listed in the warning letter are defined as drugs because they are intended to use for the diagnosis, cure, mitigation, treatment, or prevention of disease, and/or intended to affect the structure of any function of the body.

Novo Nordisk said it will continue providing Levemir vials and the Levemir FlexPen for diabetes control while supplies last, until the full product discontinuation at the end of 2024.

The FDA granted accelerated approval to Aliqopa in September 2017 for the treatment of adults with relapsed follicular lymphoma previously treated with at least two prior systemic therapies.
FDA expands indications for bupivacaine liposome injectable suspension (Exparel; Pacira BioSciences, Inc.) for use in adult patients as an adductor canal block and a sciatic nerve block in the popliteal fossa.

The global market for chikungunya virus vaccines is estimated to exceed $500 million annually by 2032.
Supplemental Biologics License Application for lisocabtagene maraleucel (Breyanzi) seeks to expand the current indication include the treatment of adults with relapsed or refractory chronic lymphocytic leukemia or small lymphocytic lymphoma who were previously treated with a BTKi and BCL2i.
Adalimumab-bwwd (Hadlima; Samsung Bioepis Co., Ltd. and Organon & Co.) is seeking to become the third Humira biosimilar deemed interchangeable with the reference product.
Adzynma is the first approved genetically engineered protein product for the treatment of patients with congenital thrombotic thrombocytopenic purpura.
FDA restricts use of pembrolizumab (Keytruda) combination in the treatment of gastric cancer to patients with certain tumor types.
Fruquintinib (Fruzaqla) is the first novel chemotherapy-free treatment option approved for metastatic colorectal cancer in more than a decade.

Tirzepatide (Zepbound; Eli Lilly and Company) is expected to be available in the United States by the end of 2023 with a list price of $1,059.87.
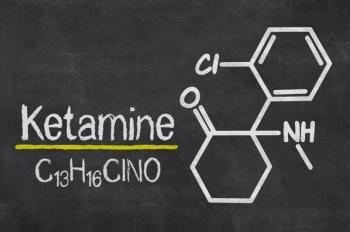
The online promotion of intravenous infusions and oral forms of subanesthetic ketamine for the treatment of mental health conditions has been found to frequently carry misleading or false information.
Due to a higher than anticipated demand, Sanofi is carefully managing distribution of 50 mg doses of Beyfortus in the private market to fulfill existing orders and provide equitable access to remaining doses for the vaccine that protects infants against respiratory syncytial virus.