
Marc O'Connor discusses healthcare services, provider integration, and more insights from the 2018 JP Morgan healthcare conference.

Marc O'Connor discusses healthcare services, provider integration, and more insights from the 2018 JP Morgan healthcare conference.



Click the title above to open the Pharmaceutical Executive January 2018 issue in an interactive PDF format.

Senior Editor looks back over the past year at Pharm Exec and offers up her favorite articles.

Killian Weiss talks to Pharm Exec about the modern challenges of identifying KOLs in oncology, and why technology can offer a solution to this vital process.

While pricing issues need to be addressed in a transparent manner, what cannot be disputed is that the vast majority of the industry is committed to the discovery of vital new medicines with the power to change patients’ lives, writes Dr Steve Arlington.



Bagrat Lalayan presents an overview of osteoporosis treatment market and offers vision for future business strategies.

The 2017 GPS ranking evaluates clinical trial registration, results reporting, clinical study report synopsis sharing, and journal article publication rates for new drugs approved by the Food and Drug Administration in 2014 that were sponsored by large drug companies.

Click the title above to open the Pharmaceutical Executive December 2017 issue in an interactive PDF format.




John Sattler offers some tips on engaging executive and professional recruiters.






Agency leaders go slow in weighing changes to DTC ads and off-label marketing. Jill Wechsler reports.


Our annual look at the drug industry's pipeline-the progress and the setbacks-puts my thoughts on those people driving new science behind the scenes in pharmaceutical research.

A deep dive into the outlook and implications from the UK’s recently unveiled life sciences blueprint for a post-Brexit world.
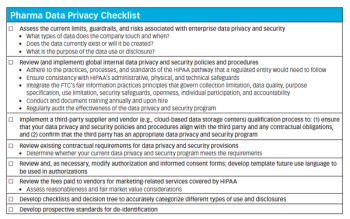
Outlining the common data transactions between life sciences companies and the HIPAA-regulated stakeholders they deal with daily-and steps pharma can take to secure and protect its own data.

Click the title above to open the Pharmaceutical Executive November 2017 issue in an interactive PDF format.



Navigating Data Privacy and Security Considerations in the Life Sciences Industry