Data from the Phase III MINT trial found that Uplizna demonstrated a greater reduction in Myasthenia Gravis Activities of Daily Living score compared to placebo at week 26.

Data from the Phase III MINT trial found that Uplizna demonstrated a greater reduction in Myasthenia Gravis Activities of Daily Living score compared to placebo at week 26.

Deal to acquire Covis is expected to expand Azurity’s portfolio across multiple complex dosage forms and key therapeutics areas.

The latest news for pharma industry insiders.
New real-world and implementation study data highlight the efficacy of ViiV’s long-acting injectables for HIV prevention and treatment, with Apretude showing zero HIV acquisitions and Cabenuva maintaining high viral suppression rates.

Jonathon Whitton, AuD, PhD, VP, auditory global program head, Regeneron, discusses regulatory challenges for DB-OTO, Regeneron’s AAV-based gene therapy for hearing loss.

A Harvard Business School Healthcare Alumni Association Q&A with Elaina Shekhter, EPAM’s Chief Marketing & Strategy Officer.

The latest news for pharma industry insiders.
Topline results from the Phase III VERITAC-2 trial found that vepdegestrant provided a statistically significant and clinically meaningful improvement in progression-free survival in patients with ER+/HER2- advanced or metastatic breast cancer.

Partnership includes the development of combination products, including a fixed-dose combination of petrelintide and Roche’s dual GLP-1/GIP receptor agonist, CT-388, for weight management.

The prospective study reportedly showed an improvement of over 10%.

Fred Aslan, MD, CEO, Artiva Biotherapeutics, explains what the FDA Fast Track designation for AlloNK in autoimmune diseases means for the acceleration of its development and potential approval of the therapy.

eHealth’s latest survey reveals Americans give the nation’s healthcare system a low grade and are open to significant changes.

The latest news for pharma industry insiders.
Results from multiple Phase III trials of Icotrokinra in moderate-to-severe plaque psoriasis and a Phase IIb trial in ulcerative colitis successfully met all primary endpoints.

Partnership is expected to support Phase III development of VK2735 in patients with obesity and metabolic disorders amid increasing demand for GLP-1 therapy.

Aliya Omer, VP, US franchise head, breast cancer, AstraZeneca, discusses Lynparza’s role in reducing the risk of invasive disease recurrence or death in HR-positive breast cancer patients.

The latest news for pharma industry insiders.

Artiva Biotherapeutics' CEO Fred Aslan, MD, discusses the concept of an immune reset and what the company is tracking in trials to assess its potential.

March is Colorectal Cancer Awareness Month, and the health plan hopes to improve survival rates with early screenings.
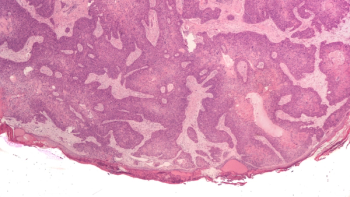
Sun Pharma’s acquisition of Checkpoint Therapeutics includes Unloxcyt, an FDA-approved anti-PD-L1 therapy for metastatic and locally advanced cutaneous squamous cell carcinoma.
Omlyclo, a biosimilar to Xolair, is indicated for the treatment of moderate to severe persistent asthma, chronic rhinosinusitis with nasal polyps, immunoglobulin E-mediated food allergies, and chronic spontaneous urticaria.

Jonathon Whitton, AuD, PhD, VP, auditory global program head, Regeneron, discusses how key regulatory designations, such as the FDA's Regenerative Medicine Advanced Therapy designation, facilitate accelerated development and commercialization of DB-OTO through increased collaboration with regulators.

Artiva Biotherapeutics' CEO Fred Aslan, MD, discusses two ongoing trials for autoimmunity indications in the US and how AlloNK differs from traditional B-cell depletion strategies.

The latest news for pharma industry insiders.
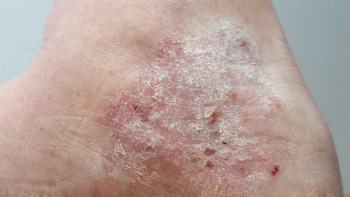
Five-year data from Phase III trials demonstrated that 67.7% of patients moderate-to-severe plaque psoriasis treated with UCB’s Bimzelx achieved complete skin clearance.

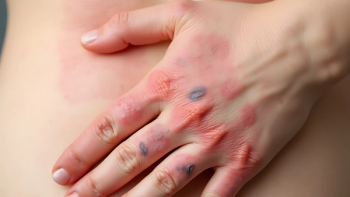
Clinical trial results found that a majority of patients with atopic dermatitis who were treated with Ebgylss achieved complete or near complete skin clearance at three years on a single monthly maintenance dose.

Jonathon Whitton, AuD, PhD, VP, auditory global program head, Regeneron, discusses the promising results of DB-OTO in the CHORD trial for children with otoferlin-related hearing loss.

The data analytics company based its new product on simple spreadsheet technology.

The latest news for pharma industry insiders.
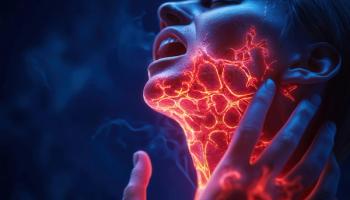
The approval of neffy marks the first major advancement in epinephrine delivery for patients over four years of age in more than 35 years.