
An overview of trends in gene therapy, the unique analytical challenges posed by developing new treatments, and innovative solutions to address these challenges.

An overview of trends in gene therapy, the unique analytical challenges posed by developing new treatments, and innovative solutions to address these challenges.
The acquisition, valued at $600 million, is expected to integrate V-Wave into Johnson & Johnson MedTech, which could improve the treatment of heart failure with reduced ejection fraction.
Approval marks the first multitargeted regimen to surpass Tagrisso in efficacy in the first-line treatment of advanced non-small cell lung cancer, according to Johnson & Johnson.

In an interview with Pharm Exec Associate Editor Don Tracy, Joseph Mikhael, chief medical officer, IMF, offers a glimpse at multiple initiatives that the IMF is working towards to improve myeloma treatment globally.

The pharmaceutical company worked with Coursera to develop the three-module course.
Approval of Niktimvo was based on positive data from the AGAVE-201 study, which demonstrated a 75% response rate in patients with chronic graft-versus-host disease.
The combination treatment involves administering Imfinzi with neoadjuvant chemotherapy for non-small cell lung cancer before surgery and as monotherapy post-surgery.

The acquisition will allow Lilly to add Morphic’s IBF treatment to its pipeline.

The test is the first of its kind and is available over the counter.
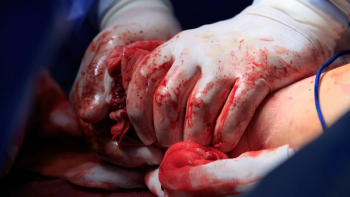
Cresilon works to stop life-threatening bleeding in seconds, offering a valuable tool for military, emergency medical services, and healthcare professionals.

In an interview with Pharm Exec Associate Editor Don Tracy, Joseph Mikhael, MD, chief medical officer, IMF, explains the goals of the inaugural Iceland Cycling Expedition.
Collaborative efforts among researchers, clinicians, and industry are essential to unlock the full potential of digital health in transforming the lives of people with Parkinson's disease.

The campaign, More to Parkinson’s, will focus on providing education about Parkinson’s related hallucinations.

Heidi Capozzi previously served as chief people officer for McDonald’s.
Approval of Livdelzi is based on results from the Phase III RESPONSE study, which demonstrated improved key biochemical markers and reduced pruritus.
UGN-102 has the potential to be the first FDA-approved treatment for low-grade intermediate-risk non-muscle invasive bladder cancer, UroGen says.
Approval of Nemluvio was based on positive results from the Phase III OLYMIA trials, which demonstrated significant itch reductions.

In an interview with Pharm Exec Associate Editor Don Tracy, Carlos Doti, VP, Head of Medical Affairs, US Oncology Business Unit, AstraZeneca, discusses a number of oncology targets in the works at AstraZeneca.

The company worked in partnership with Neurocode.

In an interview with Pharm Exec Associate Editor Don Tracy, Carlos Doti, VP, Head of Medical Affairs, US Oncology Business Unit, AstraZeneca, talks PARP inhibitors in cancer treatment and the history of Lynparza.

The Complete Response Letter stated that current data on midomafetamine capsules is insufficient for approval in treating patients with post-traumatic stress disorder.

Yorvipath is designed to parathyroid hormone exposure over 24 hours in patients with hypoparathyroidism.

Strategic imperatives for biotech firms in light of the latest market dynamics and investor behaviors.

Approval of neffy marks a significant advancement in epinephrine delivery, offering a less painful alternative to traditional needle injections.
As part of the acquisition of CN201, Curon is expected to receive an upfront payment of $700 million, with the opportunity to earn an additional $600 million in milestone payments linked to the drug's development and regulatory approval.
Lymphir is the first therapy to target the IL-2 receptor in the treatment of relapsed or refractory cutaneous T-cell lymphoma and the first FDA-approved product for Citius Pharmaceuticals.
Accelerated approval of Fabhalta for treating primary IgA nephropathy was based on positive results from the Phase III APPLAUSE-IgAN study.

Clinical trials for Crexont demonstrated a substantial increase in "Good On" time, representing a period without troubling adverse effects in patients with Parkinson disease.

The two companies will combine assets to develop a device that make automatic adjustments.

Independence Blue Cross and Jefferson health published a study detailing the impact of such care.