FDA expands indications for bupivacaine liposome injectable suspension (Exparel; Pacira BioSciences, Inc.) for use in adult patients as an adductor canal block and a sciatic nerve block in the popliteal fossa.

FDA expands indications for bupivacaine liposome injectable suspension (Exparel; Pacira BioSciences, Inc.) for use in adult patients as an adductor canal block and a sciatic nerve block in the popliteal fossa.
Eight rapid-, short-, and long-acting insulin products will move to tier one preferred status, which limits out-of-pocket spending to $35 or less for patients with diabetes.

The global market for chikungunya virus vaccines is estimated to exceed $500 million annually by 2032.
Supplemental Biologics License Application for lisocabtagene maraleucel (Breyanzi) seeks to expand the current indication include the treatment of adults with relapsed or refractory chronic lymphocytic leukemia or small lymphocytic lymphoma who were previously treated with a BTKi and BCL2i.

Bill Roth provides his thoughts on top trends impacting pharma's top executives.
Adalimumab-bwwd (Hadlima; Samsung Bioepis Co., Ltd. and Organon & Co.) is seeking to become the third Humira biosimilar deemed interchangeable with the reference product.

Blue Fin Group executive highlights how leaders can plan for immediate profitable lifecycles and beyond.
Adzynma is the first approved genetically engineered protein product for the treatment of patients with congenital thrombotic thrombocytopenic purpura.

Healthy Food Rx provides home-delivered medical prescriptions of healthy food to help address diabetes.

A look into the potential effect of psychedelic drugs on various illnesses, such as mental health conditions.
FDA restricts use of pembrolizumab (Keytruda) combination in the treatment of gastric cancer to patients with certain tumor types.
Fruquintinib (Fruzaqla) is the first novel chemotherapy-free treatment option approved for metastatic colorectal cancer in more than a decade.

Tirzepatide (Zepbound; Eli Lilly and Company) is expected to be available in the United States by the end of 2023 with a list price of $1,059.87.
Company aims to receive approval on a Type II Variation application for Sirturo for patients with pulmonary tuberculosis.
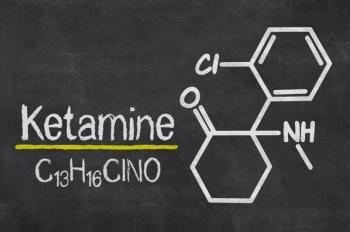
The online promotion of intravenous infusions and oral forms of subanesthetic ketamine for the treatment of mental health conditions has been found to frequently carry misleading or false information.

House Committee on Oversight and Accountability request documents on agency’s drug shortage mitigation strategies.
Due to a higher than anticipated demand, Sanofi is carefully managing distribution of 50 mg doses of Beyfortus in the private market to fulfill existing orders and provide equitable access to remaining doses for the vaccine that protects infants against respiratory syncytial virus.

Exagamglogene autotemcel (Exa-Cel) was assigned a Prescription Drug User Fee Act action date of December 8, 2023, for the treatment of sickle cell disease.

Inflation Reduction Act expected to reduce maximum achievable revenues for many top selling products.

Glyburide and metformin are active ingredients in several prescription-only medications indicated for the treatment of patients with type 2 diabetes.

While there are still hurdles to overcome along the way, home healthcare could deliver better patient-centered cancer care, providing improved patient outcomes, enhanced quality of life, and reduced strain on resources.

Healthcare organizations can lower their risk of drug diversion by strengthening staffing, employee training, and technology.

Due to a global demand, treatments such as Adderall and Ritalin have been in short supply for the management of attention-deficit hyperactivity disorder.

Incidence rates have grown for leptomeningeal metastasis, a rapidly progressing and fatal complication of several cancers.

Ongoing phase 1 dose-escalation trial of KO-2806 (FIT-001) for the treatment of patients with KRASG12C-mutated non-small cell lung cancer expected to begin dosing patients in combination with adagrasib by mid-2024.
The newly approved Alinity m high risk human papillomavirus (HPV) assay is indicated to detect HPV and for use in routine cervical cancer screening per professional medical guidelines.
Inpefa to be added on pharmacy benefit manager’s basic and high-performance formularies for commercially insured patients.

Phathom Pharmaceuticals announced that it anticipates vonoprazan (Voquenza) to be commercially available by December 2023.
SLS009 is a novel CDK9 inhibitor under investigation for the treatment of relapsed/refractory peripheral T-cell lymphomas.

Exagamglogene autotemcel (exa-cel) has shown the potential to be a landmark therapy in preventing episodes of excruciating pain among patients with sickle cell disease.