
Now officially a worldwide pandemic, the biomedical research community is rushing to develop treatments and preventives to halt the spread and severity of the COVID-19 virus.

Now officially a worldwide pandemic, the biomedical research community is rushing to develop treatments and preventives to halt the spread and severity of the COVID-19 virus.



Click the title above to open the Pharmaceutical Executive March 2020 issue in an interactive PDF format.
High customer satisfaction and low risk is the goal for patient service programs and is possible with focus on solid service delivery and quality.




The continued COVID-19 outbreak has fueled fears of drug shortages, primarily for active pharmaceutical ingredients produced in China.


With the nomination period for our latest Emerging Pharma Leaders selections in full swing, it's a good reminder that the industry's future path ultimately depends on its leaders, emerging and otherwise.
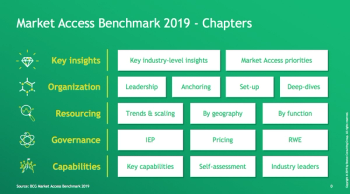
Key learnings from the BCG Market Access benchmark 2019.


Click the title above to open the Pharmaceutical Executive February 2020 issue in an interactive PDF format.

Hungary is fast staking a reputation for itself as one of Central and Eastern Europe’s most dynamic economies. A major factor has been pharmaceuticals, where Hungary’s history of research and production is reflected today in an attractive pharma investment landscape and the presence of home-grown success stories.

Guidelines on how innovators can assert patents to protect their products’ exclusivity while avoiding claims from competitors and the FTC that their conduct runs afoul of U.S. antitrust law.

Medication non-adherence is a real problem, this article looks at how to help patients better control their treatments and improve adherence.

While drug pricing remains a top topic in the media at large and worthy of continuous exploration, we shift away in our latest pharma trends report, instead spotlighting seven key themes that we think are a bit less unpredictable in the months ahead.





A summary of the key impacts new law, California Consumer Privacy Act (CCPA), will have on business practices related to data flow in specialty pharma, and more.

A list of eight points to help pharmaceutical executives focus on the role they need to play to build and maintain robust long-term partnerships.


In China’s new reform-driven and fast-moving environment for healthcare innovation, intense market pressures and fierce competition will ultimately push all stakeholders to evolve their strategies-or face extinction.

The industry faces a problem of medical education and communication in regards to vaccinations.

A look at drug pricing and patents and the role for patents and how they are under attack.

With so many different rationales for drug pricing and “value” continuing to feed the debate, the market access function in healthcare must clearly tackle these issues-but at what cost to the patient?

FDA has an official new leader, following a Senate vote to confirm Stephen Hahn as the agency’s next commissioner.