R&D/Clinical Trials
Latest News
Latest Videos

More News

LaShell Robinson, head of global feasibility and trial equity at Takeda, talks about Takeda's aims to expand its efforts globally by adapting strategies to regional needs.

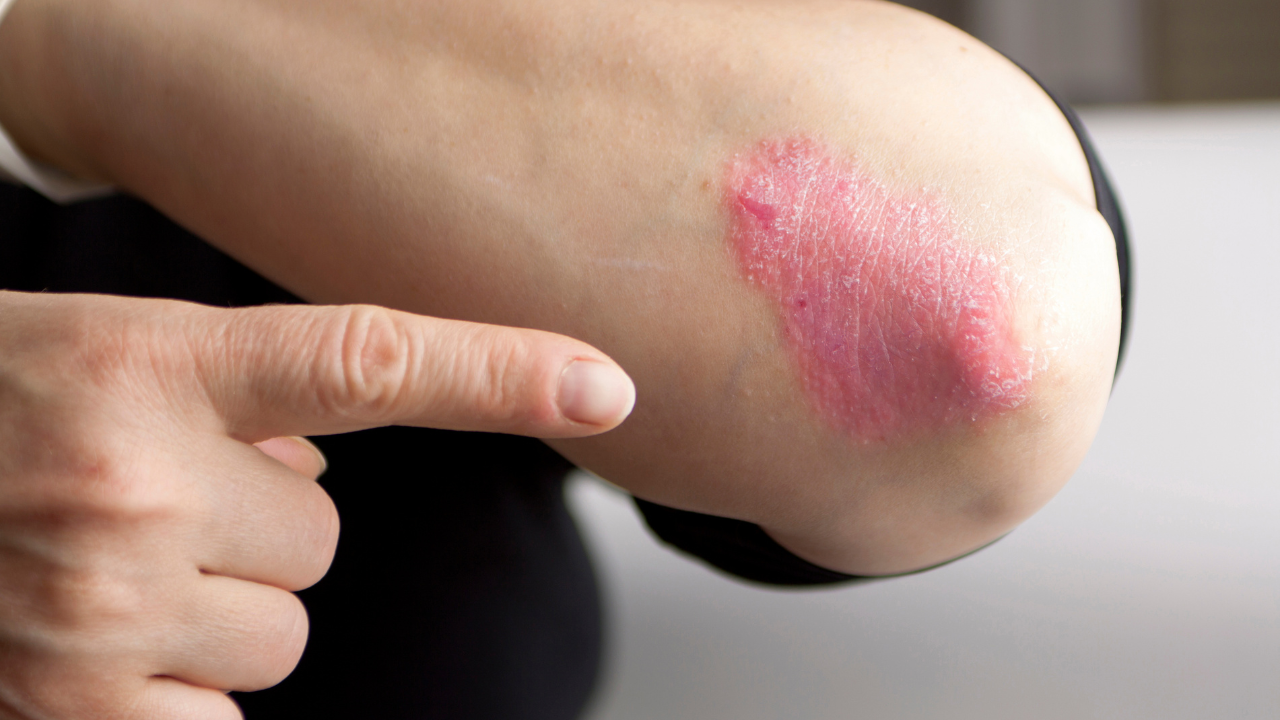
LaShell Robinson, head of global feasibility and trial equity at Takeda, discusses strategies to address underrepresentation in clinical trials, particularly in phase III psoriasis trials.

Enrollment for the Phase II trial follows FDA clearance of SPG302 Investigational New Drug application for schizophrenia.
Results from the Phase III ZENITH trial show that Winrevair lowered the risk of all-cause death, lung transplantation, and pulmonary arterial hypertension-related hospitalization by 76%.
BIIB080 marks the first antisense oligonucleotide targeting tau to enter clinical development for the treatment of Alzheimer disease.
In the SOUL trial, Rybelsus produced a 14% risk reduction of major adverse cardiovascular events in patients with type 2 diabetes and cardiovascular disease and/or chronic kidney disease.
The trial size adjustment, along with protocol modifications and the addition of higher-enrollment sites, is expected to facilitate completion of the NEPHRO CRRT study of Niyad in patients undergoing renal replacement therapy by the end of 2025.
Results from the COCOON study showed that Rybrevant plus Lacluze reduced grade 2 or higher dermatologic events by 50% compared to standard care in patients with epidermal growth factor receptor-mutated non-small cell lung cancer.
Results from multiple clinical trials showed that Tagrisso demonstrated survival benefits in EGFR-mutated non-small cell lung cancer, both as monotherapy and in combination therapies.

Jennifer Kyle, CEO and founder of Condor, discusses the inspiration behind creating a financial cloud platform specifically for biopharma R&D.
Research also showed that the test has a very low false positive rate.

The investment, which is a 25% increase from previous years, will support three new advanced manufacturing facilities, expansions at existing sites, and R&D efforts.

Phase III Trial Shows Novartis’ Gene Therapy Significantly Improves Spinal Muscular Atrophy Outcomes
Results from the STEER and STRENGTH studies showed that OAV101 IT led to a 2.39-point improvement in motor function in patients with spinal muscular atrophy.
In the Phase III STOP-HS1 and STOP-HS2 trials, results show that patients treated with povorcitinib for hidradenitis suppurativa experienced a ≥50% reduction in the total abscess and inflammatory nodule count.

Company executives provide updates on cancer treatment pelareorep and other elements of the company’s pipeline.
In the Phase III CALYPSO trial, eneboparatide demonstrated statistical significance in achieving albumin-adjusted serum calcium normalization while eliminating the need for active vitamin D and oral calcium therapy in chronic hypoparathyroidism.

Jonathon Whitton, AuD, PhD, VP, auditory global program head, Regeneron, discusses how the focus of technology development is shifting toward ensuring safety and continuous hearing for children.
Data from the Phase III MINT trial found that Uplizna demonstrated a greater reduction in Myasthenia Gravis Activities of Daily Living score compared to placebo at week 26.

Artiva Biotherapeutics' CEO Fred Aslan, MD, explains the strategic advantages of pursuing both a company-sponsored trial and an investigator-initiated trial simultaneously.
New real-world and implementation study data highlight the efficacy of ViiV’s long-acting injectables for HIV prevention and treatment, with Apretude showing zero HIV acquisitions and Cabenuva maintaining high viral suppression rates.

Immunotherapy pioneer Mark Frohlich, MD, CEO of Indapta Therapeutics, discusses the promise of harnessing natural killer cells to reshape treatment for cancer and autoimmune disease.

Artiva Biotherapeutics’ CEO Fred Aslan, MD, identifies data points the company is prioritizing to demonstrate that results from certain trials will translate to similar efficacy across a range of autoimmune conditions.
Topline results from the Phase III VERITAC-2 trial found that vepdegestrant provided a statistically significant and clinically meaningful improvement in progression-free survival in patients with ER+/HER2- advanced or metastatic breast cancer.

Dr. Dina Radenkovic, CEO of Gameto, discusses the use of iPSCs in fertility treatments, the most significant scientific hurdles they've encountered in developing and scaling these therapies (particularly in relation to ovarian aging), and how they're addressing them.
Results from multiple Phase III trials of Icotrokinra in moderate-to-severe plaque psoriasis and a Phase IIb trial in ulcerative colitis successfully met all primary endpoints.