
Donna L. LaVoie discusses four key industry areas that will experience downstream pressures in the wake of the COVID-19 crisis.

Donna L. LaVoie discusses four key industry areas that will experience downstream pressures in the wake of the COVID-19 crisis.
In 2020 and beyond, succeeding in the new ecosystem means focusing on delivering value backed by data, tailored engagements with a diverse range of distinctly defined stakeholders, and monitoring the rapidly evolving healthcare space for new trends that will impact success.

Fully understanding the construct of what it means to be lean can equip individual contributors, teams, and leaders to contribute to the fullest extent of their capabilities.

Understanding how CIOs can overcome the challenges of building a digital health Internet of Things (IoT) infrastructure is essential for harnessing digital transformation in a cost-effective and scalable manner.

Eight lessons to drive better outsourcing decisions.

Five key areas biopharma manufacturers focus on to predict where the markets will be and determine how standards of care will evolve to effectively position new products.

Mike Kean and Billy Tamulynas discuss the key components of growth-ready technology for early-stage pharma companies and how to make optimal use of them.
To ensure patients have timely access to expensive new therapies, biopharma companies have to address commercialization challenges with innovative measures, even after getting regulatory approval.

A key question for an organization post-M&A is: “What organizational capabilities support global guidance and leadership, and which of them require these at the 'local' level?” Scott Hull discusses potential solutions.

While culture may "eat" strategy, shared understanding creates both culture and strategy, write Steve Figman and Sean Robertson.
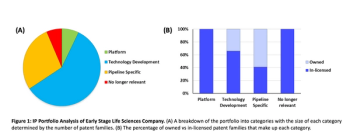
Creating an IP portfolio strategy that includes R&D, in-licensing, out-licensing, and mergers and acquisitions can help businesses stay ahead of the competition.
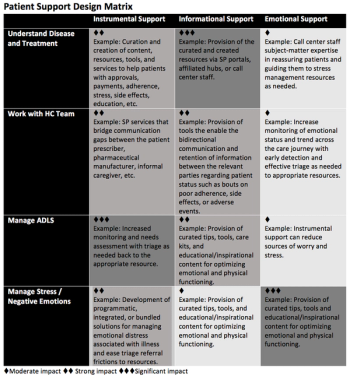
Specialty pharmacy's role in shifting the existing paradigm from a transactional model to an experiential model in ways that can fuel real-world data acquisition and strategy.

Jianan Huang discusses how the drug revenue formula is being used to guide R&D "rescue strategies".

Pair of interviews spotlight the changing dynamics for C-suite business managers in two contrasting life sciences settings-big, established pharma and small, aggressive biotech.

The significant scale that is necessary to break into the healthcare marketplace poses the greatest barrier to entry for women entrepreneurs. Shaleta Dunn outlines strategies that aspiring businesswomen can adopt to foster their success.
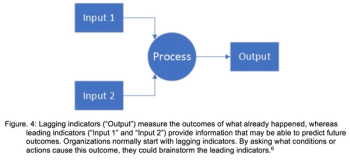
Common strategies to overcome bottlenecks and how fast they might lead to results are discussed.
In preparing to launch a rare-disease medication, it is important to understand the needs in the therapy area and invest strategically to maximize the impact on the unique rare-disease environment.

John Ebeid outlines talent strategies to reduce an organization's labor costs and increase quality.
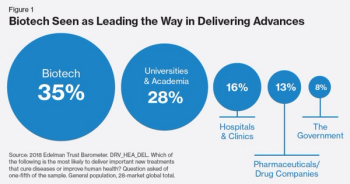
How companies can trust now to cultivate corporate reputations that build business.

Keshia Vaughn looks at how machine learning can transform commercial planning and outlines what teams will need to deploy it effectively.

New realities are transforming the global environment and upending a business model based on the presumption of ever more connected markets. Paul A. Laudicina asks, What does this new age mean for pharma?

Executives highlight approaches around recent landmark approvals for digital medicine and gene therapy.

Chad Storlie draws on military training to outline how all industries and organisations can create great junior leaders.

Commercial leaders can boost product launches with a data-driven roadmap.

Daniel Patrick discusses the small biotech company journey from an incubator of ideas to the ability to shepherd an R&D asset through its pipeline to achieving commercial success.