
Webinar Date/Time: Tue, Jan 30, 2024 10:00 AM EST | 9:00 AM CT | 3:00 PM GMT | 4:00 PM CET


Webinar Date/Time: Tue, Jan 30, 2024 10:00 AM EST | 9:00 AM CT | 3:00 PM GMT | 4:00 PM CET

JAMA study evaluates the ability of artificial intelligence to transform electronic health.

Data will continue to grow in importance in 2024.
Pharma industry stakeholders must develop actionable roadmaps to prioritize the areas in which the use of generative artificial intelligence can create the greatest benefits.

Orne discusses the forces impacting the life sciences market and how companies are reacting.

Capan discusses the industry is embracing new technologies and how these use cases may evolve in the coming year.

Emerging technologies like digital twins offer supply chain efficiency solutions, allowing the industry to integrate sustainable practices while ensuring patient safety.

Webinar Date/Time: Tue, Dec 12, 2023 10:00 AM EST | 9:00 AM CT | 3:00 PM GMT | 4:00 PM CET

The global cost of clinical research and trials is estimated to exceed $85 billion by 2030, which provides an opportunity for artificial intelligence to streamline processes.

Webinar Date/Time: Wed, Dec 6, 2023 2:00 PM EST

C-Suites and their boards should remember that digital transformation is a key part of the larger focus of a profound business transformation.

Companies must determine the right ways to utilize AI.

Webinar Date/Time: Thursday, December 7, 2023 at 10am EST | 7am PST | 3pm GMT | 4pm CET

Ito discusses how data-driven AI can be utilized by the pharma industry.

Amy West, Head of US Digital Health & Innovation Strategy at Novo Nordisk, shares her insights on fostering collaboration, embracing change, and her compelling vision to revolutionize digital transformation and innovation in the field of healthcare.

Webinar Date/Time: Tue, Nov 28, 2023 10:00 AM EST | 9:00 AM CT | 3:00 PM GMT | 4:00 PM CET

Webinar Date/Time: Tue, Nov 14, 2023 10:00 AM EST | 9:00 AM CT | 3:00 PM GMT | 4:00 PM CET

Wearable sensors and remote monitoring technology can help HCPs monitor and manage COPD more effectively.

Webinar Date/Time: Monday, November 13, 2023 at 11am ET | 8am PT | 4pm BST

Webinar Date/Time: Wed, Nov 1, 2023 11:00 AM EDT

Klein discusses how recent FDA draft guidelines will impact the use of SaMD in the life sciences industry.

How to cultivate trust & connections in life sciences in a time of AI bias.
In this Q&A with Pharmaceutical Executive®, Andrew Hopkins, founder and CEO of Exscientia, reveals how artificial intelligence (AI) is currently being utilized in the pharma industry, predictions for where AI can be implemented in the future, and what pharma fears most about AI.

Webinar Date/Time: Wed, Nov 8, 2023 1:00 PM EST
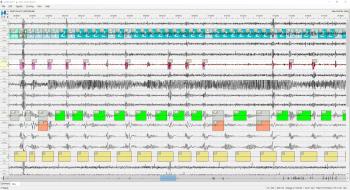
Korean-based company HoneyNaps developed the algorithm.