2014 Pipeline Report: The Sprint to Value
Across a constellation of categories, bright new drugs are moving into position. The pharma model may have changed, but companies are keeping their blockbusters.
This year's pipeline report is a little different from reports in previous years in that it takes a closer look at fewer categories – the ones where the biggest things are happening: cancer, heart failure, obesity and diabetes, and hepatitis C, in addition to novel antibiotics, not a goldmine by any stretch, but an absolutely critical need. The last category, Outsiders, Sleepers, and Early Stagers, describes products that don't fit neatly into this year's setup, but are nevertheless worthy of mention. In October, a study in Nature Reviews Drug Discovery took pharma analysts to task for getting the numbers wrong—a lot—but we've endeavored to corroborate the forecasts, both bullish and bearish, and are confident that this year's report spotlights real value, for patients and bottomlines. The pharma business model might have changed, but a lot of companies have managed to hold on to their blockbusters.

IMAGE: TILTON WIDRO
Cancer: Multiple Targets
Genome-guided tumor diagnostics and treatments with the power to manipulate a patient's immune response have the potential to impact cancer outcomes in dramatic fashion. At the Cleveland Clinic's recent Medical Innovations Summit (see Pharm Exec's "Top Medical Innovations for 2014"), Eric Klein, chair at the Clinic's Glickman Urological & Kidney Institute, championed the work of companies like Genomic Health Inc.—and its recently CLIA-approved OncoType Dx prostate cancer assay—as critical to the future of cancer research and drug development. The genotype of a tumor may prove to be more useful than the genotype of the patient in which it resides. If identifying a "driver mutation" in metastatic cancers turns out to work in practice, it won't matter where a tumor originated in the body. Consequently, "drug development will be more rapid as a result," says Klein.
Designing clinical trials around a specific tumor mutation instead of a patient's cancer type probably won't start happening next year. In the shorter term, immunotherapies have maintained their buzz among researchers, with a spotlight on the role of Programmed Death-1 (PD1) as a way to cut off one important safe harbor for cancer cells looking to protect themselves from warring T cells. In addition to Bristol-Myers Squibb's nivolumab—which received FDA Fast Track designations in malignant melanoma, renal cell carcinoma and non-small cell lung cancer last April, Stephanie Hawthorne, director, clinical, and scientific assessment at Kantar Health, points to Merck's lambrolizumab and Roche's MPDL 3280A as key players in the PD1 game. Compared with nivolumab, Roche's MPDL 3280A (which targets the PD1 ligand—PD-L1—as opposed to the receptor) may have a better safety profile, while Merck's lambrolizumab may beat nivolumab on efficacy, although it's too early for excessive confidence on that prediction, says Hawthorne.

Lambrolizumab also received FDA's Breakthrough Therapy designation last April, and clinical trials are initially focused on melanoma and non-small cell lung cancer. Nivolumab is targeting melanoma and lung cancer, with the addition of renal cell carcinoma in Phase III. Merck announced plans to study lambrolizumab in other haematolgical malignancies, and has early trials running in triple negative breast cancer, colorectal cancer, bladder cancer, and other solid tumor cancers. Thompson Reuters' Cortellis database puts sales of lambrolizumab at roughly $845 million by 2018; Decision Resources' Efua Edusei made a similar prediction, around $800 million by 2019. "Merck would definitely have a better commercial opportunity if Yervoy wasn't already on the market," notes Edusea, adding that nivolumab could eventually restrict sales of lambrolizumab. With Zalboraf also looming large in the melanoma space, the initial label, launch date, and timeline for additional indications on both drugs could open or block the entrance to blockbuster fame and revenues. Roche is taking a different approach by shifting melanoma to the backburner; instead, MPDL 3280A is targeting the biomarker population in lung cancer as a lead indication. Merck followed suit with lambrolizumab, says Hawthorne. Thomson Reuters puts nivolumab sales at just over $2 billion by 2018; Decision Resources is more conservative on nivolumab, with sales just crossing the blockbuster mark by 2019.
In some ways, J&J/Pharmacyclics' ibrutinib takes a similar approach to treating cancer by inhibiting a normally protective pathway for infection-fighting B-cells. Like cancer cells hiding from T cell lymphocytes after corrupting the PD1 receptor pathway, the B-cell receptor pathway is corrupted when B-cells turn malignant. Ibrutinib inhibits the Bruton tyrosine kinase (BTK), which regulates B-cell survival. A first in class oral BTK inhibitor and an FDA Breakthrough Therapy designee, ibrutinib has filed with FDA for chronic lymphocytic leukemia (an orphan indication), and mantle cell lymphoma, also a rare disease. Achieving that rare double win—improved survival and reduced toxicity compared with chemotherapy—plus a fast track to market in the United States has prompted analysts to go big on ibrutinib. Estimates range from $3.5 (Thomson Reuters Cortellis) to $6 billion (Credit Suisse) by 2020.
In addition to ibrutinib are Gilead's idelalisib, a phosphoinositide-3 kinase (PI3K) inhibitor, and Rigel Pharmaceuticals' fostamatinib, a spleen tyrosine kinase (SYK) inhibitor. Both products, like ibrutinib, affect the B-cell pathway. Gilead filed idelalisib in the United States for a non-Hodgkin's lymphoma indication, and in October Rigel announced an end-of-Phase II meeting with FDA to discuss fostamatinib for immune thrombocytopenic purpura, or ITP (AstraZeneca ended its partnership with Rigel after the drug failed as a treatment for rheumatoid arthritis). Thomson Reuters is bullish on idelalisib, predicting sales of $3.8 billion, just short of projected ibrutinib sales. Hawthorne gives "a slight nod" to ibrutinib due to efficacy and safety; additionally, ibrutinib is pursuing a first-line indication in addition to second-line, whereas idelalisib is not currently in the clinic for a front-line indication. Roche/Biogen Idec's obinutuzumab, a CD20 inhibitor, is also a key product in this area; tested as a monotherapy and in combination with chemo for chronic lymphcytic leukemia, obinutuzumab also plays on the B-cell pathway, and also received FDA's coveted Breakthrough Therapy designation. Obinutuzumab was also granted priority review, and given a PDUFA date of December 20, 2013. Analysts are slightly less jazzed about obinutuzumab, at least compared with ibrutinib and idelalisib; forecasts from EvaluatePharma, Credit Suisse, and Thomson Reuters ranged from $400 million to around $750 million by 2019.
In breast cancer, palbociclib, a clinical candidate emerging from a research collaboration between Warner-Lambert (now Pfizer) and Onyx (now a subsidiary of Amgen), dazzled attendees of the San Antonio Breast Cancer Conference in 2012 with the results of a randomized Phase II trial. "It quadrupled progression-free survival," says Hawthorne. Currently in Phase III, palbociclib is being tested in combination with letrozole (brand name Femara, an aromatase inhibitor), and separately in combination with AstraZeneca's Faslodex (fulvestrant), to prove superiority over Faslodex alone. Edusei says several generic products in the hormone receptor positive (HR+) space, including letrozole, are available on the cheap, but palbociclib seems to represent a significant improvement over those products. Palbociclib targets metastic breast cancer in HR+ patients—more specifically, estrogen receptor positive (ER+)—that are also HER2 negative. HER2 positive breast cancer gets a lot of press, because of many new and successful drug targeting those patients, but hormone receptor positive patients are a much larger breast cancer population: roughly 75 percent of all breast cancer incidence is ER positive, according to the University of Maryland Medical Center. Thomson Reuters predicts blockbuster sales for palbociclib; Edusei and Decision Resources puts the number at $750 million by 2019.
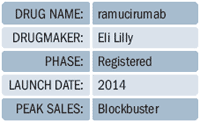
Unfortunately, Eli Lilly had to can a 1,144 patient multinational Phase III study on ramucirumab, a VEGF antiangiogenic monoclonal antibody for breast cancer in September, but there's a silver lining. Ramucirumab received an FDA Fast Track designation for gastric cancer, and a Priority Review in October. Gastric cancer is a "high unmet need...if you can prolong survival, I think [Lilly] has a very good option for those patients," says Hawthorne.Interestingly, ramucirumab targets the VEGF receptor, whereas Avastin targets the ligand. Avastin failed in Phase III trials for gastric cancer, which raises the question: is there a true mechanistic difference in targeting the receptor versus the ligand? If so, "these angiogenics will start to separate themselves in terms of where they might be active," says Hawthorne. Sanofi/Regeneron haven't announced plans to test Zaltrap in gastric cancer, but ClinicalTrial.gov shows a Phase II study of Zaltrap for esophagogastric cancer, a malignancy in the throat as opposed to the stomach. Gastric cancer incidence in Japan has risen sharply in recent years; Decision Resources says the global gastric cancer market will reach $2.3 billion by 2021, with 44 percent of those revenues coming from Japan. Thomson Reuters pegs ramucirumab sales at $725 million by 2018, Leerking Swann says $1.3 billion by 2020.
Heart Failure: Possible Success
Patients entering emergency rooms in the United States are more likely to be there as a result of acute heart failure than for any other reason. In the last 25 years, heart failure incidence has increased by about 175 percent, and what's worse is that drug development in this high need area has been abysmal, says Martin Sullivan, executive medical director of cardiovascular medicine at INC Research, and a board certified internist and cardiologist. Heart failure has been an "arena of therapeutic futility...there have been probably 20 candidate compounds tested over the last 15 years, and we're zero for 20 on these compounds," says Sullivan.

That could change with Novartis's serelaxin, a peptide hormone currently under review in the United States and Europe. FDA granted Fast Track status in 2009, and Breakthrough Therapy status last summer; Sullivan says serelaxin could be a "home run" for heart failure. Citing the RELAX-AHF Phase III data, Sullivan says serelaxin "had a reduction in dyspnea, a small improvement in [hospital] length of stay, and most importantly, a significant reduction in re-hospitalizations and death in 90 days." Serelaxin is a recombinant form of human relaxin-2, a naturally occurring hormone; pregnant women release relaxin during labor, which causes ligaments to relax and become more flexible to facilitate childbirth. Using a hormonal treatment like serelaxin for heart failure is interesting because "it has so many different effects on so many different pathways," says Sullivan. "It effects inflammation, fibrosis, cell cyclin, it's a vasodilator, it does all kinds of things."
It could also make Novartis the proud parents of a new blockbuster for acute heart failure. Thomson Reuters predicts sales of roughly $1.2 billion by 2019, and serelaxin could receive European approval by this year's end. Seamus Fernandez, managing director of major and specialty pharmaceuticals at Leerink Swann, wrote in a recent analyst note that serelaxin's Breakthrough Therapy status did not confer an expedited FDA review of the drug, according to Novartis management, but the company expects a US approval in late 2014. Leerink Swann is predicting $1.3 billion in sales for serelaxin by 2020.
Sullivan also points to Acorda Therapeutics GGF2 candidate—a naturally occurring neuregulin or growth factor—and the results of an early stage trial, which improved some patients' ejection fraction by nine percent. Ejection fraction is a measure of how effectively the heart pumps blood. Like serelaxin, GGF2 is a peptide product with multifaceted effects in the body, including "cell cyclin, cell death, CNS effects...and it effects cell programming a little bit, potentially in vivo," says Sullivan. After a 24-hour infusion, patients given a higher dosage of GGF2 showed a heart function improvement at 28 days and 90 days. Acorda said it has discussed a new trial protocol with FDA for 2013, presumably a Phase II study, but ClinicalTrials.gov shows only a small Phase Ib double-blind study testing a single GGF2 infusion for safety and tolerability. This product may be further off, but Sullivan says if the data holds through Phase III, "that would be a major advance in heart failure."
Incidentally, the Chinese company Zensun is also testing a neuregulin drug candidate called Neucardin for chronic heart failure. A Phase II trial has been completed in the United States, and drug has been filed already in China. The company is currently enrolling a Phase III trials in the United States, but Phase II data was less impressive than Acorda's. However, a Zensun press release states that 678 people with chronic heart failure have been given Neucardin, and Phase II data demonstrated a "three to five percent placebo corrected improvement in left ventricular ejection fraction." SciClone Pharmaceuticals will market Neucardin in China, in a deal worth upwards of $30 million if Zensun meets its regulatory milestones. Forecasting for GGf2 and Neucardin is too speculative at this point to be meaningful, but if any drug candidate shows it can improve symptoms and survival in heart failure patients, the dollars will almost assuredly follow.

Device Alert
Last year's pipeline covered the PCSK9 inhibitors—notably Amgen (AMG 145) and Sanofi/Regeneron's (alirocumab) candidates, both of which have important Phase III data read-outs in 2014—but there isn't much new to report since last year aside from forecasts on both drugs getting reined in. Leerink Swann now estimates that AMG 145 won't quite reach blockbuster status by 2020, and Thomson Reuters scaled back alirocumab to just under $500 million by 2019. Credit Suisse still thinks AMG 145 will reach blockbuster status by 2020, and puts alirocumab's sales at $865 million in 2020. During its 3Q earnings report in late October, Pfizer announced a "major" Phase III program for its PCSK9 inhibitor, called bococizumab, according to an analyst note from Fernandez at Leerink Swann. Three is a crowd, so Pfizer tossing its hat into the PCSK9 ring can't be good news for Amgen and Sanofi/Regeneron.
Obesity and Diabetes: Two Birds One Stone
There's still a lot to be said for the American Dream (assuming it hasn't succumbed to heart failure), but the American Diet is not worthy of admiration. "Other countries are starting to live like us...and die like us," said Dean Ornish, president of the Preventative Medicine Research institute, during the Cleveland Clinic's recent conference on obesity and diabetes. "Weight loss can reverse heart disease, prostate cancer, Type 2 diabetes" and other conditions, he said.
FDA has already approved two new obesity drugs—Arena/Eisai's Belviq, and Vivus's Qsymia—the first FDA approvals for the condition in over 15 years, but given the size and extent of the health problem, not to mention the cost, better treatments and devices are needed. Future therapies will attempt to target the "microbiome" or gut flora, to change the way that food is synthesized in the gastrointestinal tract. Anthony Viscogliosi, principal at Viscogliosi Brothers, a New York-based VC/private equity firm, said he wants to invest in "meds that inhibit fat syntheses...that can change the microbiome to turn sugar and fat into water."

Next-gen probiotics and nutraceuticals could potentially impact the microbiome—scientists have developed a trimethylamine N-oxide (TMAO) assay that detects TMAO—a microbial byproduct of intestinal bacteria—in the blood, that is associated with a 2.5-fold increase in stroke and heart attack. But the microbial genome, or microbiome, contains about 3.3 million genes. Compared with what we still don't know about the human genome, and its 23,000 genes, an active therapy for a specific microbiome target probably won't emerge in the next couple of years. However, Steve Nissen, the Cleveland Clinic's department chair of cardiovascular medicine, told conference-goers that the TMAO biomarket "could take off in 2014...and drugs will follow."
In the shorter term, Orexigen Therapeutics may be the most promising and lucrative late-stage oral medication for the treatment of obesity. In January, FDA asked for additional cardiovascular outcomes data and proposed a resubmission procedure for Orexigen's Contrave, a naltrexone/bupropion combination pill, and the company expects to resubmit to FDA by the end of this year. Orexigen appears to have faired better in Europe; EMA has accepted its submission under the centralized procedure, and Orexigen expects approval in the second half of 2014. Despite a similar efficacy in comparison with Belviq and Qsymia, naltrexone/bupropion is still getting blockbuster nods. Thompson Reuters projects roughly $1.1 billion by 2019, and Credit Suisse corroborates that approximation, putting 2020 sales at $1.1 billion. Credit Suisse predicts very similar sales for both Belviq and Qysimia, which suggests the level of need in the market, or opportunities for the products to differentiate from one another, or both.
Novo Nordisk is preparing to file a 3-mg version of its GLP-1 liraglutide (aka Victoza) for an obesity indication in the United States, and has entered Phase III trials in over 20 countries for that indication. In the United States, Novo will file by the end of this year, and expects to get an approval in 2014. The company may try to best the new raft of obesity treatments by maximizing the label; in June, Novo completed a Phase III trial studying obese patients with sleep apnea. Clinical studies indicate that liraglutide at 3 mg could bring down body weight by an average of eight percent, a slight improvement on Belviq and Qysmia, but liraglutide is an injectable, not a pill, and it's expected to be costly. Gideon Heap, an analyst with Decision Resources, says he likes liraglutide in obesity for the following reasons: "In theory, [liraglutide] will avoid any of the CNS side effects that trouble a lot of physicians in obesity, and Novo also has some good trial designs going," says Heap. "They are developing it with a Phase II trial that attempts to show prevention of the onset of Type 2 diabetes. If that gets a positive outcome, I think it would represent a strong bargaining chip for getting better reimbursement [over Belviq and Qsymia]." Credit Suisse forecasts have liraglutide's obesity indication adding another $500 million a year to the increasingly lucrative Victoza franchise, by 2019. Decision Resources predicts roughly $800 million by 2019, with a launch late next year.
Since 1997, there have been 10 new classes of diabetes drugs, and yet, many patients still aren't able to keep their glycemic levels under control. Takeda hopes to bring an 11th class of drug to market with its TAK-875 compound, a GPR40 agonist. Currently in Phase III testing, TAK-875 is going head-to-head against Januvia, and could potentially launch in 2015 or 2016, according to Heap. TAK-875 "is causing a bit of excitement because it's showing good efficacy while maintaining a high level of safety, of a kind you would see with the DPP4 inhibitors," says Heap. "It's as efficacious as sulfonylurea, but in Phase II showed low rates of hypoglycemia, and crucially, it doesn't appear to induce any kind of weight gain."

Device Alert
Al Mann hopes to bring a new drug delivery device to market; this time he's more hopeful than ever. In September, Mann personally assured Pharm Exec that Afrezza, a product that needs little introduction, will receive its long-awaited FDA approval in April—if it isn't approved somewhere else first. Analysts seem to agree that MannKind's inhalable insulin will indeed get approved, but a lingering question remains: will patients want to use it? "There aren't a lot of patients out there demanding an inhalable insulin," says Heap. "Possibly, the developers of these [inhalation] devices are a bit carried away with how important they think that is." After completing two 24-week Phase III trials with its next-gen Dreamboat inhaler device, MannKind resubmitted with FDA in October. Thomson Reuters is predicting over $2 billion in sales, but Kiran Meekings, a consultant at the Thomson Reuters Life Sciences team, isn't so sure. She worries about the potential for adverse pulmonary effects over the long term. MannKind's clinical work has attempted to mitigate concerns about lung function associated with chronic use of an inhaled insulin, and Mann says Afrezza "is not in the lungs very long, we go quickly through the membrane into the blood, with no accumulation." Meekings says MannKind's pulmonary safety study results "weren't necessarily cause for concern...but it's something that needs to be monitored."
Finally, Eli Lilly and Boehringer Ingelheim are attempting to bring a biosimilar insulin—the oldest biologic drug—to market. The European Medicines Agency (EMA) accepted the companies' long-acting insulin glargine biosimilar submission in July, and the product is in Phase III in the United States. Insulin glargine, also known as Lantus, is Sanofi's top seller, with over $6 billion in sales last year. Credit Suisse is bullish on the biosimilar version, with predicted sales topping $1.2 billion by 2019, not bad for a copycat. Thomson Reuters is more conservative, with a forecast of $415 million in annual sales for the biosimilar by 2019. Sanofi, for its part, is rushing forward on U300, a reformulated insulin glargine; the company said during a 3Q conference call that it would file in the United States and Europe during the first half of 2014. That product's new attributes, according to Sanofi, are fewer incidences of hypoglycemia, and a smaller volume of subcutaneous injection compared with Lantus. Incidentally, Lantus loses patent protection in 2015. Novo Nordisk, the biggest seller of insulins worldwide, hopes to wrest back marketshare from Sanofi, but probably won't get Tresiba approved in the United States before 2016, at the earliest. Thomson Reuters estimates the new and improved version of Lantus will earn $1.1 billion by 2019, a fraction of Lantus's current annual sales.
Hepatitis C: Banking on Sofosbuvir
Ben Weintraub, senior principal and director of research at inThought, a division of Symphony Health Solutions, calls his $3 to $4 billion sales estimate on Gilead's sofosbuvir—a nucleoside analog polymerase inhibitor targeting all six HCV genotypes—conservative. "The question is not whether sofosbuvir is going to be the biggest drug for hepatitis C ever, or the biggest drug launch ever...I think everyone agrees that it's going to be both of those things," says Weintraub. "The question is whether it's going to be the biggest drug ever."
Weintraub says he's seen estimates as high as $15 billion, but the reason for his conservatism is the misguided assumption that the population of hepatitis C patients receiving treatment will rise from a current level of 10 percent, to as much as 90 percent once interferon, and it's horrid side effects, are taken out of the picture. Weintraub thinks the percentage of HCV patients receiving treatment will only increase to around 20 percent. The price of therapy is one access-limiting element, but more importantly, says Weintraub, is the fact that a substantial portion of the HCV population won't all of sudden come in to the clinic for sofosbuvir, or any other HCV drug. "If you're an IV drug user, if you're homeless and you have HCV, getting treated for it is not your primary concern," says Weintraub. "Your depression, your alcoholism, your drug abuse, your 12 other diseases, those are the things that doctors might be more worried about taking care of first."
Several analysts including Weintraub say sofosbuvir will receive FDA approval by the end of this year. Sofosbuvir will be prescribed in combination, but Weintraub says it's likely to be priced per regimen, not per drug, at "whatever the market will bear, $70,000 for sofosbuvir, riboviron and a protease inhibitor, whatever that winds up being."
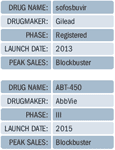
Raghuram Selvaraju, managing director and head of healthcare equity research at Aegis Capital Corp., says Enanta/AbbVie's ABT-450 combo is "every bit as good as sofosbuvir," but isn't getting as much press because the combination regimen will likely be three or four drugs, instead of potentially two with sofosbuvir. Selvaraju says both sofosbuvir and ABT-450 "are very, very good at treating genotype-1 treatment naïve patients"—the largest HCV genotype globally, and the hardest to treat—"but they are much less good at treating null responders." After the initial fanfare surrounding sofosbuvir's "marquee drug launch," Selvaraju says the quad ABT-450 regimen "is going to show better activity against null responders with genotype 1 than the sofosbuvir two-drug regimen." Phase III data from two ABT-450 studies are scheduled to read out this quarter, but the product isn't expected to launch until 2015. However, FDA gave ABT-450 Breakthrough Therapy status in May, which doesn't automatically confer an expedited approval, but could possibly tip approval into 2014. On a recent AbbVie earnings call, CEO Rick Gonzalez told investors that ABT-450's interferon-free studies are "coming in April 2014." Scott Brun, AbbVie's head of drug development, dismissed an analyst questions about "pill burden" associated with the ABT-450 regimen, mentioned above by Selvaraju. "Sustained virologic response is king, and we're in the high 90 percent range," said Brun.
Hepatitis C is heating up as these drugs and others compounds get closer to market, but the key late-stage players haven't changed. Recently approved in Japan, J&J's simeprevir is expected to be approved in the United States by this year's end, and BMS's daclatasvir picked up FDA's Breakthrough Therapy designation in April. For more information on these products and others in HCV, see Pharm Exec's 2013 Pipeline Report. Weintraub describes J&J's simeprevir as an Incivek/Victrelis follow-on drug, and says with AbbVie and BMS both launching in late 2014 or 2015, "Gilead looks like it will have a least a year" without meaningful competition. But there's a caveat: the initial sofosbuvir approval includes interferon; the all-oral HCV combo everyone is waiting for won't get approved until 2014.
Novel Antibiotics: HHS Begs for New Treatments
Most large pharmaceutical companies haven't rushed to develop new superbug-swatting antibiotics or anti-infectives, despite a dire need for such products. That's because historically, antibiotic R&D programs are often long and expensive, without much of a financial upside on the other end; prices have been pushed down by generics, and the course of therapy is short. The pricing problem could change; in September, the CDC announced that some 23,000 people in the United States die from antibiotic-resistant bacteria each year, and at least 2 million are infected. "There's a very direct correlation between superbugs and incredibly high hospital costs and mortality, says Thong Le, managing director of WRF Capital, the VC arm of the Washington Research Foundation. "A lot of the antibiotics we're using today have been around for 50 years."

Last year's Generating Antibiotic Incentives Now (GAIN) Act, ratified as a provision PDUFA V, is designed to push drug makers back into the antibiotic and anti-infective space. Thanks to the GAIN Act, incentives for companies willing to take up the task of novel antibiotic development include automatic eligibility for Fast Track status and Priority Review, which shortens the FDA review period to six months. Once approved, the antibiotic would be granted an additional five years of market exclusivity.
It's not clear whether that's a strong enough kick to make Big Pharma roll. Last spring, HHS's Biomedical Advanced Research and Development Authority announced a deal with GlaxoSmithKline to provide up to $200 million over five years to develop new antibacterial products. Similar government-financed deals with Big Pharma have been struck in Europe. Meanwhile, a few smaller biotech firms are focusing on the antibiotic-resistant and infectious disease space, and several new products could go to bat against the superbugs next year.
Raghuram Selvaraju at Aegis Capital Corp. says Cubist, with its dual acquisitions of Trius Therapeutics and Optimer Pharmaceuticals in 2013, has positioned itself "in the vanguard of anti-infective drug development." Fast Tracked via the GAIN Act, Trius's tedizolid phosphate, a second-geneartion oxazolidinone antibacterial agent was filed with FDA in October for the treatment of acute bacterial skin and skin structure infections, and is also being tested for the treatment of serious Gram-positive infections, including those caused by methicillin-resistant staphylococcus aureus (MRSA). The drug will compete against Pfizer's Zyvox, with a couple of added benefits, like easier administration and dosing, and slightly fewer side effects. Credit Suisse has modest estimates for tedizolid, $200 million by 2020. A second Cubist product, CXA-201, in Phase III for complicated intra-abdominal infections and complicated urinary tract infections, is expected to file at the end of this year, assuming the Phase III data is strong. Selvaraju thinks CXA-201 will launch in early 2015, and earn $700 million by 2022. That product will face off against meropenem, a broad-spectrum beta lactam antibiotic to which bacteria is growing resistant.
The best targets for infectious disease, from a financial perspective, are community-acquired pneumonia and complicated skin and skin structure infections (CSSSI), says Selvaraju. "We like CSSSI because the clinical trial design is pretty straight forward, compared with a lot of other indications," says Selvaraju. "We like community-acquired pneumonia lung infections because there are comparatively fewer solutions for that currently available."
Selvaraju says Cempra's solithromycin, a Phase III product targeting community-acquired pneumonia, shows promise. In September, Cempra reported in vivo data showing that solithromycin is active against a broad range of pathogens, including four of FDA's Qualified Infectious Disease Pathogens under the GAIN Act.
For the moment, pharma doesn't seem to be answering Janet Woodcock's increasingly frantic calls for new novel antibiotics. Many small companies, including Cellceutix, Durata, Anacor, and others are moving through clinical trials and targeting serious bacterial infections, but more work is needed to better understand the complex biology of the superbugs, and to take them down.
Outsiders, Sleepers, and Early Stagers
Like last year, some of the products uncovered during the research phase of this year's pipeline report weren't easily shoehorned into the categories presented, for one reason or another. Here are few category outsiders, sleepers—defined as potential big sellers people aren't talking about—and early stage drugs that look solid, but haven't gotten far enough in the clinic to prompt blockbuster whispers.

» Amorcyte's (acquired by NeoStem in 2011) AMR 001 drug candidate, an early-stager, is a bone marrow-derived stem cell product in development for the emergency treatment of severe heart attack. The company is assessing CD34-positive autologous stem cells (autologous means the stem cells come from the same patient they're used to treat) that would potentially improve health outcomes following a severe heart attach. Results from the Phase II trial are expected during the first half of next year. Martin Sullivan at INC Research says next-gen cell therapies like AMR 001 represent the next generation of better cell therapies, a promising development
» Novartis's secukinumab is an Isle 17 monoclonal antibody for the treatment of psoriasis. A sleeper because psoriasis is often in the shadow of rheumatoid arthritis, at least from a biologic drug perspective, secukinumab could wake up investors with blockbuster sales as soon as 2018, according to several forecasts. In Phase III trials, Novartis had to toss out the psoriasis area and severity index (PASI) 75 scale, because everyone in the trial passed the 75 percent mark, says Ben Weintraub, at inThought.
» Even as an early-stager, Oramed Pharmaceuticals' ORMD 0801 is ahead of the big diabetes shops in the race to oral insulin for diabetes. Oramed is pursuing a Type 2 indication first, for patients who don't want to transition to injected insulin, but the company has also launched a trial studying ORMD 0801 in Type 1 insulin dependent patients. Raghuram Selvaraju, at Aegis Capital Corp., says if ORMD 0801 finds its way to market in the next four or five years, "it would blow [MannKind's] Afrezza away." Liquid (and Afrezza's powedered) insulin must be kept refrigerated; an oral version, particularly without the need to keep it cold, could be huge in markets with access issues.
» Merck's suvorexant, a hypnotic and outsider, has been filed in the United States and Japan for the treatment of insomnia. But FDA denied the application last summer in a Complete Response Letter, over worries that the dosage was too large. Merck plans to resubmit in the first half of 2014; FDA did not require additional trials. The consensus among analysts prior to the CRL was blockbuster sales, by 2019. Given the relatively short hold up in resubmitting, Merck may still get a sweet dream or two out of suvorexant.
» GW Pharmaceuticals' sleeper product Sativex is probably the first drug derived from raw cannabis to cross the FDA's desk at least since marijuana was slapped with a schedule 1 classification in 1970. Schedule 1 drugs are considered to have "no currently accepted medical use." Sativex is approved in 22 countries, but in the United States, Phase III trials for a cancer pain indication will conclude next year, with an NDA to follow in early 2015, according to GW Pharma CEO Justin Gover. Credit Suisse puts sales at roughly half a billion by 2020.
» Synageva Biopharma's sebelipase alfa is an outsider in Phase III for the treatment of Wolman disease, a rare lisosomal storage disorder in which patients are unable to break down certain lipids. Most infants with the disease don't survive more than a year, and adults with the disease have problems with swelling of the abdomen, liver enlargement and serious, life-threatening digestive problems. Sebelipase alfa would become the first approved treatment for Wolman disease, and FDA has granted Orphan Drug, Fast Track and Breakthrough Therapies status. Given the pricing environment for orphan drugs, Synageva Biopharma could be rewarded handsomely.
Ben Comer is Pharm Exec's Senior Editor. He can be reached at bcomer@advanstar.com.
Forecasting data in Pharm Exec's 2014 Pipeline Report relies in part on Springer's Adis R&D Insight database, Thomson Reuters Cortellis, Credit Suisse, and Evaluate Pharma data. We very much appreciate the use of these resources.
Cell and Gene Therapy Check-in 2024
January 18th 2024Fran Gregory, VP of Emerging Therapies, Cardinal Health discusses her career, how both CAR-T therapies and personalization have been gaining momentum and what kind of progress we expect to see from them, some of the biggest hurdles facing their section of the industry, the importance of patient advocacy and so much more.
Applying Porter’s Five Forces to Portfolio Management in Pharmaceutical R&D: A Strategic Roadmap
March 17th 2025The increasing costs and complexity of R&D in the pharmaceutical industry have necessitated the adoption of strategic portfolio management to optimize resource allocation and enhance competitive advantage.
