AIDS in Africa: The Road Forward
If the Aspen model works, it could change the model for the developing world. Research-based companies could focus on R&D, and leave the manufacturing to generic companies.
In 1999, The world was banging on the industry's door for free AIDS drugs. With one hand, activists pummeled companies' intellectual property, their prices, and their access programs, and with the other, painted antiretroviral (ARV) drugs as the single magic bullet for solving the worst scourge to ever face mankind.

Views from Uganda's Queen Elizabeth National Park, Uganda.
The idea that free AIDS drugs wasn't the answer fell upon deaf ears, and the industry struggled to define its role in what was essentially a gruesomely massive public health and economic problem. Out of those troubled waters grew corporate social responsibility programs of unprecedented scope. Companies took a radical approach: instead of free drugs—an unsustainable donation given the scope of the problem—they wanted to invest in African systems that could grow and multiply and allow governments to treat their own people.
That year, Bristol-Myers Squibb (BMS) implemented its ambitious $115 million public health plan that reflected the enormity of the task at hand. The program, Secure the Future, targeted nine African countries—places like Burkina Faso and Lesotho—to begin developing the infrastructure needed to respond to and cope with the pandemic.
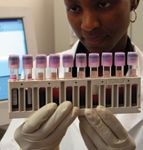
A lab technician at work in an African clinic funded by Secure the Future.
Secure the Future inspired other companies to go forward with their initiatives—and they did. The industry responded to AIDS, malaria, and tuberculosis with more than $2 billion in aid in 2003, surpassing the annual budgets of development partners like the World Health Organization (WHO) or UNICEF, according to the Hudson Group. Many of those programs, like BMS', were five-year commitments. That means company initiatives now stand at a critical point, where their futures are being decided.
Last month, Pharm Exec offered a snapshot of ARV distribution in Africa and the obstacles organizations face in obtaining drugs. This article discusses the road forward—how companies are developing new models, scaling up programs, and teaming up to fashion a better tomorrow for Africa.

Views from Uganda's Queen Elizabeth National Park, Uganda.
The Aspen Experiment
Back in 2001, Dr. Peter Mugyenyi was busy making secret arrangements to bring the first shipment of generic ARVs into Uganda. After researching doses, quality and cost, he decided to purchase the drugs from the Indian generic manufacturer Cipla. However, Cipla balked at the request: At the time, 39 drug companies were fighting the government of South Africa in court, contesting what they felt was their right to compulsory license ARV drugs. Mugyenyi wasn't a licensed importer, but he told Cipla to send the drugs anyway—he would take full responsibility for the consequences.
Mugyenyi never landed in court. But his actions, and those of others like him, helped create a new era of intellectual property laws and mores in treating AIDS. Later that same year, companies dropped the court case in South Africa and began to license ARVs to generic manufacturers for use in the developing world. GlaxoSmithKline (GSK) gave South Africa's Aspen Pharmacare permission to manufacture and distribute Retrovir (zidovudine), Epivir (lamivudine), and Combivir (zidovudine/lamivudine). The following year, Boehringer Ingelheim (BI) issued Aspen a license to produce Viramune (nevirapine).

BMS chief executive Peter Dolan meets residents of the KwaZulu Natal Province, South Africa, in an AIDS awareness event.
Those licenses would prove vital in the next round of debate over access and patents, which came when President Bush's Emergency Plan for AIDS Relief (PEPFAR) was implemented in 2004. The program, a five-year $15 billion initiative to combat HIV/AIDS in 15 target countries, aims to increase ARV usage, especially in Africa. But the US government said PEPFAR must buy FDA-approved products—there was to be no quality double standard for rich and poor patients. They also said that generic ARVs would be eligible for purchase only where they did not violate patent rights. The sum of that equation meant PEPFAR used brand-name drugs only.
The situation changed in January 2005, when FDA granted tentative approval (given when patents or marketing exclusivity are still in effect in the US) to Aspen's co-packaged offering of lamivudine/zidovudine and nevirapine.
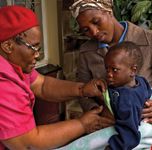
Some industry initiatives include home-based care as part of larger community-based programs for rural populations.
Activists hailed the approval as a major advance. They believe local manufacturers can produce AIDS drugs cheaper than brand-name companies because they have lower labor costs and because they've learned efficiency by working with low-margin products. Mark Isaac, vice president of the Elizabeth Glaser Pediatric AIDS Foundation, estimates the co-packaged treatment's price at $16 to $20 a month, less than half of the $55 "no profit" price that he pays for the same drugs from the research-based industry. The drugs will also be cheaper because they are exempt from the 10 percent tariff recently imposed by the East African Community's Customs Union on imported medicines, which was intended to stimulate regional trading.
If the Aspen model works, says one Big Pharma company source, it could change the model for the developing world. Research-based companies could focus on R&D, and leave the manufacturing to generic companies. But the truth is, some serious questions remain about the Aspen model:

In It Together
Cheap drugs Some executives doubt that the Aspen model will actually deliver low prices. They remember President Bill Clinton's HIV/AIDS Initiative, which promised to obtain steep discounts on ARVs by working closely with generic manufacturers. However, at a meeting with NGOs and the Indian generic firms in January, many procurement professionals say they haven't been able to obtain the "Clinton price."
Supply issues Just because a company produces drugs cheaply, consistent supplies are not guaranteed.
"Generics are usually available today, but tomorrow you're not sure," says Joseph Saba, CEO of Axios, a consulting firm that implements the industry's programs in Africa. "People who work with generic usually end up buying brand drugs while waiting for deliveries of generic," he says.

Forecast the Future
Brazil and Thailand have both called on the pharma industry to supply ARVs when copy manufacturers couldn't fulfill their tenders. But in Africa, where the demand is so great, pharma might have a difficult time picking up the slack. Without better coordination of supply efforts, those hiccups can pose a real and present danger for patients that the drugs won't arrive as planned.
Quality control Companies have to dedicate in-house resources to ensure that licensees maintain the promised quality. The big concern is that poor-quality drugs will lead to increased resistance. Secondary concerns are that low-quality licensed generics might dilute brand value or harm companies' reputations.
Market dynamics As with any new market dynamic, executives involved with licensing must rewrite existing business rules. GSK, for example, licensed several AIDS patents to Kenyan-based Cosmos, but is just now negotiating what the generic's name will be.
There are also questions about how both brands will work in one market. "Here we are licensing for exactly the same territories and indications for which we are supplying and marketing our product," says Jon Pender, GSK's director of global access issues. "It's new for us to do it this way."
Intellectual property Although licensing (and the Aspen deal) give the impression that pharma's intellectual property is being respected, there are strong signs that it will not be for long. Marie-Paule Kieny, director of WHO's Initiative for Vaccine Research, recently stated that, when an HIV vaccine is finally developed, it will be made widely available irrespective of patent rights.
That may sound like a promise to patients. But to private-sector companies, it's a warning. Some research suggests that the push for access is causing companies to silently back away from AIDS research. According to American Enterprise Institute's Roger Bate, 80 companies conducted AIDS-related research five years ago. Today, only 60 do. Some of that might be explained by industry consolidation, but the policy environment probably plays an important role as well.
Promising Models
Merck went into Botswana in 2000 with good reason. In this small, relatively well-off and politically stable country, 38 percent of adults were infected with AIDS. The government had stalled, unable to combat the the situation with few hospitals, clinics, or professionals trained to treat AIDS patients—they hadn't even mapped out how they planned to fight the epidemic. In short, if there was ever a place to test public/private partnerships to combat AIDS, Botswana was it.
What resulted is the African Comprehensive HIV/AIDS Partnership (ACHAP). Merck pledged $50 million and free drugs over five years and the Bill and Melinda Gates Foundation donated another $50 million, to kick-start a response to AIDS in that country.
Brad Ryder, ACHAP knowledge support manager, says the program gets at the heart of knowledge transfer: "What if you helped governments, gave them enough resources, and made HIV drugs free of charge? How would that country then go about fighting, tackling, and turning around the AIDS epidemic?"
As of December 2004, 28,000 people in Botswana received ARVs as part of ACHAP's free drug program. That's a huge accomplishment. But for Merck, and other companies doing development work in Africa, the greatest challenge lies in leveraging the learnings of individual programs into a much broader anti-AIDS strategy.
Sustainable, scalable, replicable Although the number of patients on ARVs is growing (next year more than a million patients will access those drugs in Africa), it's no match for the increasing rate of infection. In that context, it would be easy to dismiss pharma's demonstration programs as irrelevant "islands of excellence." But, aside from treating people today, these initiatives provide treatment models for tomorrow.
"Pharma initiatives should be sustainable, scalable, and replicable," says Trevor Neilson, executive director of the Global Business Coalition on HIV/AIDS, which leads corporate involvement in the AIDS crisis. "My biggest fear is companies will say, 'We don't have much to show for these five years except the people we served and the programs we built. We haven't been able to replicate. We haven't been able to scale up,'" he says.
Neilson worries less about drug and medical supply donation programs than about cash-heavy programs like BMS'.
Secure the Future's John Damonti, president of the BMS Foundation. echoes those concerns. "At the conclusion of the community support programs, there can't just be six well-operated communities on HIV/AIDS. Those have to be models for larger expansion across the country."
So what models allow for rapid expansion? One that has gained attention is the Baylor International Pediatric AIDS Initiative, which includes a network of ten clinics. The concept dates back to 1996, when Mark Kline, MD, of the Baylor College of Medicine visited Romania and saw the desperate plight of children with AIDS there. With funding from Abbott's Step Forward program, Kline rebuilt the crumbling Constanta Municipal Hospital. And within just a few years, mortality rates in HIV-positive children dropped from 15 to three percent. The clinic worked so well that Abbott funded centers in Tanzania, Burkina Faso, and India—and expects to announce another clinic in Africa shortly.
"Mark's work in Romania could be considered an UNAIDS best practice case study," says Reeta Roy, divisional vice president, global citizenship and policy at Abbott. "We must create as many Romanias with as many people as possible to create the strongest impact."
Working Together
Back in 2000, the Accelerating Access Initiative (AAI) brought together competing companies to figure out how to reduce the price of ARVs in nations hardest hit by AIDS. Today, almost half of all patients that access ARVs in Africa do so through AAI programs—a testament to what companies can do when they work together. Other organizations also broker company relationships:
Partnership for Quality Medical Donations Twenty-one organizations, including 10 pharmas, comprise this membership organization that is dedicated to raising the global standards for product donations.
Global Business Coalition on HIV/AIDS This group focuses on bringing business into the fight against AIDS and includes a special section for healthcare companies.
Companies have also been creating alliances on their own. Merck went into Romania early on to establish a foundation to the fight: their funding helped health authorities understand the scope of the disease and develop Romania's first national AIDS treatment guidelines. Abbott leveraged that groundwork by funding Mark Kline's pediatric AIDS clinic there. Then, BMS decided to build two new clinics in Africa, working closely with Abbott to replicate the Romanian model's success.
Abbott has also worked closely with BI, in a venture where BI donated Viramune and Abbott donated the Determine rapid HIV test. "That's an example where each company had a tool to help NGOs cut the risk of mother-to-child transmission of AIDS," Roy says.
Despite these joint efforts, there is still more work to be done. "Companies work with groups based on the relationships they cultivate," says Dickson Opul, MD, who treats the HIV-positive working population in Uganda. "We don't see the effort as AAI, but as individual companies."
To push programs to the next level, companies must work together to create common strategies and shared world views. In doing so, they can secure their places as both innovators and global citizens by offering help to policy makers, who must tackle even bigger, more systemic issues like poverty reduction and free trade.
Navigating Distrust: Pharma in the Age of Social Media
February 18th 2025Ian Baer, Founder and CEO of Sooth, discusses how the growing distrust in social media will impact industry marketing strategies and the relationships between pharmaceutical companies and the patients they aim to serve. He also explains dark social, how to combat misinformation, closing the trust gap, and more.
Pfizer, GSK Gain ACIP Recommendations for RSV and Meningococcal Vaccines
April 18th 2025The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted to expand access to Pfizer’s respiratory syncytial virus vaccine Abrysvo for high-risk adults in their 50s and voted in favor of GSK’s meningococcal vaccine, Penmenvy, for streamlined adolescent protection.
