The Brands of Yesteryear: Where Are They Now?
We check back with previous Pharm Exec Brand of the Year winners to see how things are going.
Pharm Exec's Brand of the Year selections from the last few years are still alive and kicking, although some have aged more gracefully than others. Combined, PE's picks pulled in nearly $30 billion in 2013, which complicates the oft-repeated notion that the blockbuster drug era has come to an end. Patent expiry isn't even the end for some biologics and other complicated products in the US, given the challenging regulatory environment for biosimilars. The US drug pricing wars may be coming, but these products already made their mint. We checked back in with them to see how things are going, and to look for indicators about their future prospects.
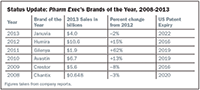
Status Update: Pharm Exec’s Brands of the Year, 2008-2013
Last spring, FDA launched a review of incretin mimetic drugs, including Januvia, following published research suggesting the possibility of increased pancreatitis. Although FDA said patients should continue on therapy, and didn't ultimately add new safety information to Januvia's label, the publicity may have been partly responsible for the small decline in revenues. That and the launch of J&J's Invokana, the first of a new class of drugs called SGLT2s. Another SGLT2, Farxiga, went live in January.

In 2013, Humira brought in more sales revenue than any other brand. Abbvie, Humira's parent after the Abbott Laboratories divorce, will continue to pursue additional indications for the product; currently, Humira is being tested in a pivotal Phase 3 trial for fingernail psoriasis in patients with moderate-to-severe plaque psoriasis. In the first quarter of 2014, Humira earned $2.6 billion, more than half of that sum arriving from markets outside the US.
Gilenya's nearly $2 billion in sales last year aren't as strong as some analysts initially thought they'd be by now, due to a combination of cardio-related safety concerns and competition in the category. A patient death spooked physicians and regulators, even though FDA found nothing conclusive linking Gilenya to patient deaths. On the competitive side, Biogen Idec priced its Tecfidera product, another oral medication, just below Gilenya when it was approved last year. But Gilenya looks like it's headed in the right direction.
The release of Medicare payments made to doctors last month caused a minor imbroglio that, among other things, demonized ophthalmologists for prescribing Lucentis instead of Avastin for wet macular degeneration. Avastin isn't FDA approved for that use, but studies point to similar efficacy rates, and Avastin is available at a much lower cost than Lucentis. That may seem like a positive development for Avastin, but it's actually a net negative; Genentech (which markets both drugs) will face more criticism over its maneuvering with Avastin and Lucentis, a money game without much regard for patients or costs to the healthcare system.

Probably the last of the blockbuster statins, Crestor has had a pretty good run, considering it was late to the party. AstraZeneca was able to extend its patent on Crestor in the US, until 2016, but had less luck in other countries, where it's already available in generic form. But Crestor is hanging in there, and grew sales to $1.3 billion in the first quarter of 2014, a 2% increase from the first quarter of 2013.

Beleaguered smoking cessation drug Chantix has become something of a pop culture nightmare, due to the strange and occasionally violent behavior sometimes seen in patients who take the drug. There's now a "Chantix defense" in court, most recently deployed by Tim Danielson, who allegedly murdered his ex-wife. Pfizer said blood tests show Danielson didn't have Chantix in his system at the time of the murder, but Danielson says the drug's side effects, including depression, played a role in the crime.

Ben Comer is Pharm Exec's Senior Editor. He can be reached at bcomer@advanstar.com.
Applying Porter’s Five Forces to Portfolio Management in Pharmaceutical R&D: A Strategic Roadmap
March 17th 2025The increasing costs and complexity of R&D in the pharmaceutical industry have necessitated the adoption of strategic portfolio management to optimize resource allocation and enhance competitive advantage.
Cell and Gene Therapy Check-in 2024
January 18th 2024Fran Gregory, VP of Emerging Therapies, Cardinal Health discusses her career, how both CAR-T therapies and personalization have been gaining momentum and what kind of progress we expect to see from them, some of the biggest hurdles facing their section of the industry, the importance of patient advocacy and so much more.
