Country Report: Romania
After several years on the back burner, Romania’s pharmaceutical sector appears to be enjoying a resurgence-one it hopes will catapult the country’s healthcare system on a path to sustainability over the long haul.
This sponsored supplement was produced by Focus Reports.
Project Director: Louis Haynes
Coordinator: Valerie Baia
Project Assistants: Zachary Burnside, Angelo Basurto
Report Publisher: Mariuca Georgescu
For exclusive interviews and more info, please log onto www.pharmaboardroom.com or write to contact@focusreports.net
WHERE CREATIVITY BRINGS PROFITABILITY
Once touted by the IMS as one of the "fast followers" among the world's "pharmerging" nations and feted by the international investor community in the wake of EU accession, Romania's pharmaceutical industry appears resurgent once more after a number of years on the back burner.

(LLUSTRATION CREDITS: CARMEN REYES)
In 2014, Romania's market size increased by a full 6.8 percent to EUR 2.76 billion [USD 3.12 billion] with Romanians purchasing OTC drugs to the tune of some EUR 755 million [USD 853 million] in the final quarter alone, reflecting an 11.8 percent year-on-year rise, according to market research conducted by Cegedim. Meanwhile the country's newly appointed minister of health has been both audacious and energetic in radically reshuffling the rules of the game to place public healthcare provision "firmly on a pathway to sustainability."

Nicolae BÄnicioiu, minister of health
“A corner has been turned and we are well on the way to establishing the enabling environment for an enduring healthcare system that is viable over the long run and can still accommodate the reimbursement of the latest generation of innovative treatments,” explains Minister Nicolae BÄnicioiu.
Such optimism chimes with a national economy enjoying a sharp rebound from the instabilities of the global financial crisis. Indeed, signs of economic health abound. Romania is currently registering an admirable 2.7 percent GDP growth rate, placing it as the sixth best performing economy of the European Union, and saw its FDI ratio leap 45 percent this year. Meanwhile the country’s exceptional record on fiscal consolidation continues to generate acclaim. “The current account deficit has narrowed to a mere 0.5 percent making Romania a stellar performer,” exclaims IMF chief economist Ana Babici.

RÄzvan VulcÄnescu, secretary of state, Ministry of Health
What’s more, the overwhelmingly positive economic trajectory shows little sign of abating, with the latest European Commission forecasts predicting four percent year-on-year GDP increases by 2018. “Romania heralds a thriving economy, geographical proximity to Western Europe and a labor cost of only 30 to 40 percent of Berlin’s. You’re staring at enticing production opportunities and an attractive trading partner with disposable income on the up and the average income increasing by around six percent annually,” enthuses Sebastian Metz of AHK, the German Chamber of Commerce.

CÄlin GÄlÄÅeanu, BMS country manager
The core attractiveness of the Romanian pharmaceuticals market derives from its potential as growth patterns in mature markets continue to flatten and Big Pharma embarks on a quest for alternative revenue sources. “Romania generates interest because you have a demographic profile that situates the country as the seventh biggest EU population with some 20-odd million inhabitants,” observes BMS country manager, CÄlin GÄlÄÅeanu. “Other compelling characteristics constitute the prevalence of certain infectious diseases such as TB where the affliction rate is abnormally high and HIV/AIDS where there is a very specific cohort of patients.”

Fabrizio Giombini, MSD managing director
“If you calculate the opportunity for market increases in Western Europe, there is frankly not so much scope for selling more. The plateau has already been reached. Yet Romanian pharma, by contrast, offers much more mileage. Taking into consideration the fact that per capita consumption here lags at roughly half of neighboring Hungary, then this is clearly an exciting prospect where the ‘boom period’ is still waiting to happen,” concurs Bogdan Savu, country manager of Gerot Lannach.

Dr Marius Savu, president of NAMMD
USHERING IN THE BRAVE NEW WORLD OF ROMANIAN HEALTHCARE
No one can accuse the incumbent government of being lethargic when it comes to healthcare reform. After an unprecedented seven years of zero new molecules being admitted to the state reimbursement list, the pharma industry has suddenly been subjected to a whirlwind of radical refashioning and legislatory upheaval. “The industry has changed more in the past six months than it has in the entire previous five years,” says Nolan Townsend, country manager of Pfizer Romania. “Such a pace of overhaul can, though, yield some nice results.”

Nolan Townsend, country manager, Pfizer
Fabrizio Giombini, managing director of MSD Romania, mirrors that sentiment. “Introducing originators to the local market has been a real challenge for innovative pharma companies. Eventually, last year, we finally made some tangible progress towards a new reimbursement system, though there remains considerable ground to cover,” he warns.

Senator Florian Bodog, secretary of the Health Commission
“I believe that we’ve achieved a great many milestones within a very tight timeframe. We’re talking about the reopening of 26 hospitals, 3,500 new jobs for health practitioners and a new list of subsidised medicines, not to mention the introduction of a national health card and new national programs for urgent unmet needs,” declares Minister BÄnicioiu.

Vasile Ciurchea, president of CNAS
“Not only did we introduce 40 new molecules last year – a feat simply unimaginable a few years ago – but also we have implemented an entire supporting architecture to enable similar updates to be conducted on a regular basis in the future: namely a delisting mechanism, cost-volume agreements and health technology assessment (HTA) capabilities,” emphasizes secretary of state at the Ministry of Health, RÄzvan VulcÄnescu.

Barbara Cygler, general manager, GSK
Marius Savu, director of the National Agency for Medicines and Medical Devices (NAMMD), also identifies a decisive turning point. “Radical new concepts such as HTA and managed access agreements pave the way for biannual introduction of innovative medicines. Our expectation is that this new regulatory landscape will become the definitive model for many years to come.”

Gabor Sztaniszlav, Amgen general manager
Not everyone is so unequivocally optimistic, however, about Romania’s ability to propel itself forward in terms of healthcare outcomes. “The problem is that, in Romania, healthcare is all to often considered from a purely economic standpoint: how much is spent on medicines. But the indirect social consequences should be factored in as well. If life expectancy remains one of the lowest in Europe or if the number of days spent on sick leave by workers remains abnormally high then this is going to harm the economy as a whole and translate into reduced productivity and a lower tax intake. Under such circumstances the end balance is a negative one,” argues Gabor Sztaniszlav, general manager of Amgen Romania. “We believe that there must be more public money injected into the system.”
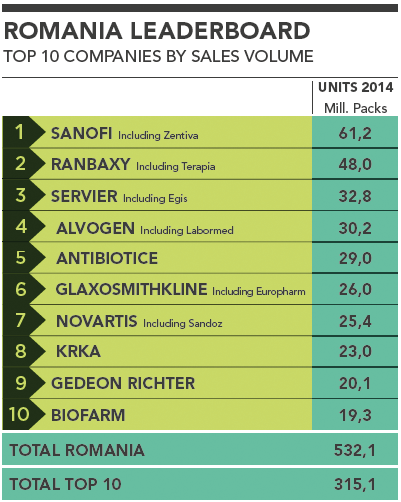
Romania Leaderboard
Indeed, according to the European Health Consumer Index, which objectively evaluates the healthcare systems of the EU28 alongside a further eight non-EU European members, Romania now ranks 35th out of the 36 counties analyzed, with only Bosnia Herzegovina faring worse. “If you want a healthy economy you certainly need to invest money in healthcare because people constitute the main asset of a country,” affirms GSK’s general manager for Romania, Barbara Cygler. “The entire local industry has been built up in barely 20 years with the rapid emergence of a young medical class with high expectations, but we’re still waiting for that greater cultural paradigm shift that assigns healthcare its true worth as an investment in the nation,” reasons Roberto Musneci, senior partner at Serban and Musneci Associates (SMA).

Petru CrÄciun, Cegedim general manager
“This is really problematic,” says Mark Dekker, Astellas’ country manager for Romania, “because unfortunately, there’s really no other substitute for introducing new liquidity into the system; none of the new mechanisms being embraced by the state will be able to overcome such a basic underfunding shortfall irrespective of how well they are applied.”

Dan Zaharescu, executive director ARPIM
UNTANGLING THE GORDIAN KNOT OF CHRONIC UNDERSPENDING
Such assertions touch upon one of the seemingly intractable issues afflicting Romanian healthcare: chronic underfinancing. “If you scrutinize the KPIs and macro indicators, Romania lags well behind its peers both in terms of drug consumption per capita and national expenditure on healthcare,” points out BMS Romania’s country manager CÄlin GÄlÄÅeanu. Indeed, “an annual spend hovering around the four percent of GDP mark places the country more on a par with Madagascar and Burundi, than any European counterpart,” notes Amgen Romania’s Sztaniszlav.

Doru Torge, Ferring general manager
The state, however, remains impervious to such appeals. “I think that if we don’t have the means to regulate spending, to control any wastage of money, and if we don’t envisage healthcare prevention, then even were the government to hypothetically direct a full ten percent of GDP towards healthcare, then we would find that even that would not be enough. The real problem lies elsewhere and relates to the rationalization of how we deploy the resources at our disposal,” counters Minister BÄnicioiu.

Practical Strategies For Generating Profits In A Hostile Regulatory Climate
“We certainly want government health spending to grow, but have to consider the impact at the macro level so that an overall budget equilibrium can be maintained. Successive governments have noticed that, regardless of how much money has been allocated directly to healthcare, we have not been seeing a return on the investment. While the expenses increased year-on-year, results did not follow accordingly which indicates a financial wastage inherent to the system that first needs resolving,” says Senator Florian Bodog, secretary of the Health Commission in the upper house.
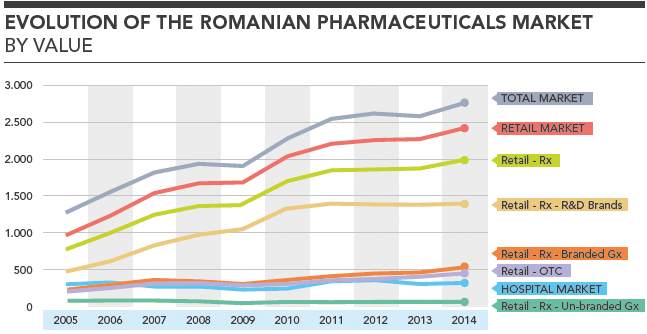
Evolution of the Romanian Pharmaceuticals Market
Meanwhile the verdict of the IMF would appear to be strikingly similar. “An important prerequisite to be able to increase the public spend would be to put some order in how the system functions, because we are confident there is ample room within the existing envelope to make significant savings and efficiency gains which could then be directed towards financing new drugs. If you have a car losing gas, do you put more gas into it or instead first try and fix the leak?” muses Ana Babici.
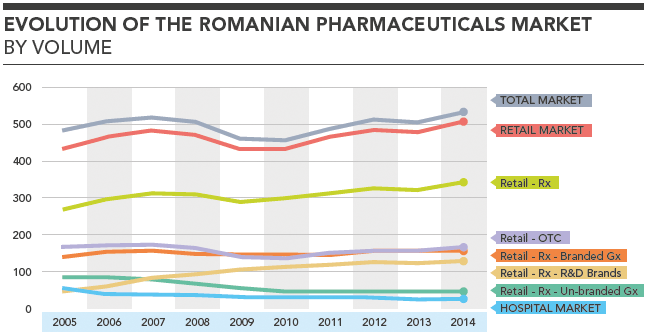
Evolution of the Romanian Pharmaceuticals Market
“This is precisely why we set about rolling out the national health smartcard,” asserts Vasile Ciurchea, president of the National Health Insurance House (CNAS), the state institution overseeing public healthcare expenditure. “This year the health card has become operational alongside existing digitalization mechanisms such as e-prescriptions with the direct purpose of better targeting health spending. It guarantees there is no drainage, loss or misallocation and validates the provision of each specific medical service. Writing fictional prescriptions and selling the drugs elsewhere will now become much harder,” he reasons.

Adrian Grecu, Mylan general manager
“What’s more, the fact every citizen will have their own electronic dossier backed up on the health card will enable us to conduct data mining and extrapolate information for the nation as a whole which can then be used to inform better healthcare policy making,” attests secretary VulcÄnescu. “The only problem here is that there’s no silver bullet or switch to be flicked… with all of these structural steps forward, there’s always a hefty time lag before the fruits of the endeavor become apparent and patients see the end benefits.”

Laurenţiu Mihai, APMGR executive director
Though the utter gulf between Romania’s present level of healthcare provision and EU averages might initially appear overwhelming, many remain optimistic that catching up can be swift. “Being a latecomer can actually be an advantage if you learn from the experiences of others and avoid the missteps,” reminds Ferring’s general manager for Romania Doru Torge. “There can be benefits from a leap-frog effect.”

Simona CocoÅ, general manager Zentiva
NAVIGATING A COMPLEX OPERATIONAL ENVIRONMENT
The historic healthcare underspend and ongoing strict cost-control orientation of successive administrations naturally impacts the operational environment. “Healthcare and the pharmaceutical markets work hand-in-hand synergistically and need to grow congruently for both sectors to flourish,” says Petru CrÄciun, general manager of Cegedim Romania. “The adverse effects of a stagnating healthcare system are beginning to spill over into the pharmaceutical market and cause complications for the industry.”
Mobilizing A “Coalit¸ia” For Combined Progress
These issues manifest themselves in a variety of ways. First and foremost is the widely despised “clawback mechanism”, essentially a sales tax on prescription drugs that is adjusted to cover whatever shortfall occurs between the government budget for medicines and actual consumption. Secondly, the state enforces mandatory price controls with pharmaceutical price tags referenced to the lowest denominator of a basket of 12 EU, but not necessarily Eurozone, markets.

Radu Cazacincu, Magistra C&C management board member
“Developments with the clawback and mandatory pharmaceutical pricing have been causing a real headache for our members,” explains Dan Zaharescu, executive director of the Association for Innovative Pharmaceutical Manufacturers (ARPIM). “By far the most difficult element to swallow has been the ballooning of the clawback mechanism from 20 to 26.4 percent despite the market appearing relatively flat. The lack of transparency and arbitrariness over how the contribution is calculated is causing havoc with our members’ balance sheets and damaging their ability to engage in strategic planning,” he claims.

Vijay Palamadai, country manager, Dr. Reddy's
Many, however, view factors such as the clawback more as symptomatic of deeper structural issues rather than inherently problematic in itself. “Contrary to what you might suppose, the clawback is not unique to Romania,” explains Jacopo Murzi, general manager of Janssen Romania. “Successful attempts to contain costs via an analogous mechanism have been deployed in Hungary, Bulgaria and Poland. The difference in all those instances, however, is that the clawback was simultaneously used as a way to fund expensive novel therapies. Here in Romania, we are completely missing that side of the equation. It’s being utilized solely as a device for meeting current budget requirements with no forward-looking perspective.”

Voices From The Inside
BACK TO THE FUTURE: REPRIORITIZING BRAND BUILDING
Another curious feature of the Romanian market is the relative lack of generics penetration. “Generics currently hold around 44 percent local market share and 24 percent of the value. Unfortunately we have actually been witnessing a decrease in presence over the last few years which is highly unusual for a market such as ours, with so many unmet needs, when generics could potentially be providing a cheaper alternative to innovative drugs,” bemoans Simona CocoÅ, general manager of Zentiva.

Roberto Musneci, managing partner SMA
For many players, the solution seems to be about weighting portfolios towards OTC, branching into supplements and brand-building. “Romanian generics is at a strategic inflection point: on the one hand, there are industry forces such as price pressure, a tough regulatory environment, shifting demographics and increasing patient empowerment. On the other hand, the digital revolution affords us a new tool to create, deliver and communicate value. We have to prove capable at adapting to the evolving landscape around us,” muses RÄsvan Baltag, country manager of Medochemie Romania
Quite why generics are gaining so little traction remains a topic of intense debate. “This is less to do with consumer preferences and more about the behavior of the pharmacists who are forced down a path of favoring the sale of expensive branded products in a bid to shore up their cash registers. The sad reality is that many Romanian citizens cannot familiarize themselves with generics when large volumes of the cheapest drugs are being forced out of the market by a very hostile fiscal regime dominated by the claw-back mechanism,” declares Laurent¸iu Mihai, executive director of the association for generics manufacturers (APMGR) pointing to statistics demonstrating that no fewer than 1,300 of the cheaper generics have disappeared from the market since 2011.
Others are convinced that there are sociocultural forces at play. “It may be connected to our historical legacy under a communist regime when we did not possess quality labeled products, but Romanians are undoubtedly a brand conscious people. After all, Romanians don’t talk about trainers or photocopies, but ‘Adidas’ and ‘Xerox.’ Brands are fixed in the national consciousness,” expounds Mylan Romania’s general manager, Adrian Grecu.
Glenmark’s area head for the Balkans, Lavinia Oprea, supports this reasoning. “Unbranded generics tend to perform poorly despite their favorable price differentials, while in the prescription market, originators are still king of the game… this is because consumer tastes in Romania are very orientated towards brands and launching a product very cheaply doesn’t necessarily fly as a strategy,” she explains.
The end effect produces some surprising about-turns in company strategies with certain players jettisoning the conventional approaches for CEE markets in favor of a return to brand building. “Previously we were pursuing the usual strategy of building up a portfolio, but increasingly found market demand challenging this thinking,” recollects Vijay Palamadai, country manager of Dr. Reddy`s Romania. “This may be of some surprise to the pharmacists and distributors who think we are trying to swim against the tide, but the reality is that this market is only deceptively a generics market,” he concludes.
CONTRACT MANUFACTURING PROWESS
If multinationals have been struggling to adapt their Romanian strategies, one area of the industry that appears to be flourishing is the local production segment. Much of this impetus relates directly to EU accession and the harmonization of manufacturing standards. “You can describe it as a short, sharp shock, but once we found ourselves aligned with international norms, the business opportunities were phenomenal. Having GMP certification opened the door to a whole world of new possibilities hitherto unknown,” recalls Radu Cazacincu, from the management board of the family-owned, Constant¸a-based local producer Magistra C&C.
“The switchover inevitably produced a certain shakeout across the industry with a number of the smaller and less secure players disappearing entirely from the scene. Those that managed to adapt to the new rules of the game, however, were soon rewarded with a whole new host of foreign partnerships and international contracts,” concurs Attila Santa, founder of the Brasov-based local manufacturer Sanosan.
Future trends would seem to favor Romania’s rise as a manufacturing outsourcing leader. “If ten years ago contract manufacturing agreements were finding their way over to India and China, these days, as EU regulations tighten, the logistical limitations of these arrangements have become increasingly apparent and the entire business model requires a rethink,” explains Santa.
“Countries like Romania are actually the primary beneficiaries,” agrees Cazacincu. “The nation’s local producers offer competitive and more affordable prices compared to the rest of the EU and yet our quality standards are equivalent to and, in some cases, superior due to the modernity of our factories. This ties in very well with a willingness to withdraw contract manufacturing networks from Asia and re-situate them within Europe,” he adds.
“From an economic perspective there is also a strong case for leveraging countries located on the fringes of the EU like Romania for product conditioning and customization because of the attractive cost differentials. The EU comprises 27 states of very different sizes, each with their own market specificities and these needs require segmenting. Romania in many respects offers a platform for last-minute customization of the batches. The smaller the batches being prepared, the more the overheads become pronounced and it’s in instances like this when the cheaper, more flexible, but highly skilled Romanian labor pool really comes to the fore,” explains Santa.“We certainly see a niche emerging for small and medium size local producers such as Magistra C&C,” admits Cazacincu. “Our size and geographical proximity means that we are flexible enough to respond to the evolving demands of our clients at pretty short notice.
Some multinationals are already starting to arrive at the same conclusions. “We realized that the educational profile of the workforce, low labor costs and flexible labor code all combine to make a compelling case for ramping up investment in local manufacturing and service support centers,” admits Pfizer Romania’s Nolan Townsend. “We are actually diverting product lines from elsewhere and placing them in Romania to make full use of what we see as a real competitive advantage not just vis-à-vis Western Europe, but Central Europe as well,” he reveals. “Conceptually, Romania should definitely become a manufacturing hub for the EU and epicenter of European export-orientated production,” suggests Dr. Reddy’s Romania’s Vijay Palamadai.
ON THE BRINK OF A TIPPING POINT…
One senses that Romanian healthcare is on the cusp of a new era. From within the ministries and state apparatus, the mood is bullishly confident. For pharma industry actors, well used to false dawns and creatively having to identify new pockets of profitability in a fluid policy context, attitudes are more tempered. “I would say Romanian healthcare is enduring growing pains and it’s really not entirely clear what exactly will emerge from all this policy renewal,” conjectures Cegedim Romania’s Petru CrÄciun.
What is certain, though, is that changes are afoot that could well prove long-lasting. “Double digit market growth is here to stay, patients are becoming conscious of their rights and local manufacturers are assuming new roles in Europe-wide networks…these are certainly fascinating times,” concludes SMA’s Roberto Musneci.
