
In a Phase I trial, data suggest that Amgen's maridebart cafraglutide (MariTide) may allow patients to take lower and less frequent doses over time while still maintaining significant weight loss.

In a Phase I trial, data suggest that Amgen's maridebart cafraglutide (MariTide) may allow patients to take lower and less frequent doses over time while still maintaining significant weight loss.
Patients with relapsed or refractory multiple myeloma administered Blenrep combined with bortezomib plus dexamethasone experienced a 59% reduction in the risk of disease progression or death compared with the standard of care.
The report predicts a 77% increase in global cancer cases by 2050.
BioNTech SE and Duality Biologics' next-generation antibody-drug conjugate is being evaluated for patients with platinum-resistant ovarian epithelial cancer, fallopian tube cancer, or primary peritoneal cancer previously administered one to three systemic treatment regimens.
The FDA assigned a Prescription Drug User Fee Act action date of June 7, 2024, to an application that would expand the indication of Arexvy to include adults 50-59 years with an increased risk of respiratory syncytial virus-related lower respiratory tract disease.
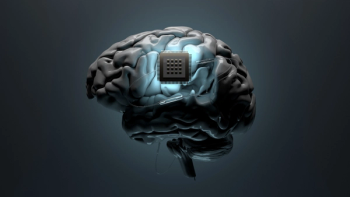
Synchron’s brain-computer interface device is being developed to allow patients with mobility challenges to operate certain technology with their mind.
UV1 plus ipilimumab (Yervoy) and nivolumab (Opdivo) produced a statistically significant and clinically meaningful survival improvement in patients with unresectable malignant pleural mesothelioma.
The $1.3 billion acquisition—which included two aqua manufacturing plants—expands Merck’s portfolio in the veterinary pharma space.

By capitalizing on AI advancements, pushing the boundaries of personalized medicine with theranostics, and strengthening digital healthcare networks, radiopharmaceuticals are poised to transform healthcare from a reactive to a proactive discipline.
Higher prices of insulin attributed to significant annual increases, outpacing general inflation.

The era of big pharma as product-first companies must end, as services become the larger priority.
The drugmaker will directly acquire three finish-fill sites as part of the deal.

In an interview with Pharm Exec Associate Editor Don Tracy, Shubh Goel, Head of Immuno-Oncology and Gastrointestinal Tumors, US Oncology Business Unit, AstraZeneca, provides her thoughts on the success of the trial.
Beyfortus (nirsevimab-alip), a monoclonal antibody that protects against respiratory syncytial virus-associated lower respiratory tract disease, experienced higher than anticipated demand that led to shortages during the 2023-2024 season.

The agency issued the warning against three brands of unapproved eyedrops designed to look like an approved brand.

Novartis rescinds rights to to develop and commercialize multi-tyrosine kinase inhibitor dovitinib due to a material breach by Allarity for lack of financial payment.
The biologics license application for afamitresgene autoleucel, an engineered T-cell receptor drug, was assigned a PDUFA date of August 4, 2024.
BST02 is the first tumor-infiltrating lymphocyte therapy for the treatment of all types of liver cancer to have advanced to the clinical trial stage.
The US Department of Health and Human Services has initiated a comprehensive strategy to address the escalating syphilis rates, as a result.

DELFI-Tumor Fraction assay was developed to improve noninvasive assessment of tumor burden and monitoring of treatment efficacy and resistance in patients with advanced cancers.
Rusfertide is currently in a pivotal Phase III clinical trial as a potential first-in-class treatment for polycythemia vera.
Vabysmo is the first bispecific antibody approved to treat ocular conditions such as diabetic macular edema and wet age-related macular degeneration.

App aims to leverage digital technology to significantly reduce pharmacy costs.
Aduhelm (aducanumab-avwa) was granted accelerated approval by the FDA in June 2021 despite misgivings from the agency's Peripheral and Central Nervous System Drugs Advisory Committee.

In an interview with Pharm Exec associate editor Don Tracy, Lynn Kirkpatrick, PhD, CEO, Ensysce Biosciences, discusses the FDA's Breakthrough Therapy Designation for PF614-MPAR, which focuses on protecting patients from drug overdoses.
Darzalex Faspro has previously been approved by the FDA for eight indications in multiple myeloma.
Approval sought for Padcev (enfortumab vedotin) plus Keytruda (pembrolizumab) for the first-line treatment of adults with previously untreated locally advanced or metastatic urothelial cancer, which would be the first approved combination alternative to platinum-containing chemotherapy for this patient population.

Eli Lilly advertisement emphasizes that advancements in in medicine can lead to improved population health.

Researchers noted the onset of symptoms in patients who were treated with contaminated HGH therapy.

CAR T-cell therapy Breyanzi (lisocabtagene maraleucel) to be evaluated in patients with relapsed or refractory follicular lymphoma and mantle cell lymphoma following treatment with a Bruton tyrosine kinase inhibitor.