Adjuvant treatment with Moderna’s mRNA-4157 (V940) in combination with Merck's Keytruda lowered the risk of recurrence or death by 49% compared with Keytruda monotherapy.


Adjuvant treatment with Moderna’s mRNA-4157 (V940) in combination with Merck's Keytruda lowered the risk of recurrence or death by 49% compared with Keytruda monotherapy.
Welireg (belzutifan) approved for the treatment of adults with advanced renal cell carcinoma that progressed after a regimen of a PD-1 or PD-L1 inhibitor and a VEGF-TKI.

Eflornithine (Iwilfin) approved by FDA for adult and pediatric patients with high-risk neuroblastoma who showed at least a partial response to previous multi-agent and multimodality treatment that included an anti-GD2 immunotherapy.

MAPS Public Benefit Corporation filed a new drug application for MDMA (midomafetamine capsules) for use with psychological intervention for the treatment of post-traumatic stress disorder.

Current industry challenges present an opportunity for innovation and the adoption of new solutions, particularly in addressing barriers related to patient recruitment, engagement, and retention.

Bristol Myers Squibb will pay $800 million upfront to SystImmune for the rights to codevelop and sell a potentially first-in-class bispecific antibody-drug conjugate that has shown promise treating non-small cell lung cancer and breast cancer.
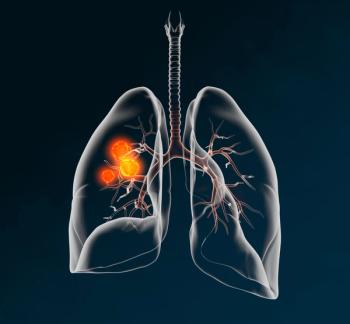
Phase III trial to investigate novel individualized neoantigen therapy V940 (mRNA-4157) in combination with Keytruda (pembrolizumab) as an adjuvant treatment for patients with completely resected Stage II, IIIA, or IIIB non-small cell lung cancer.

Lack of trust in pharma-provided content proves to be a key barrier to successful engagement, highlighting issues like promotional spin, information availability, and more.

Cresemba is now the only azole antifungal treatment approved by the FDA for use in pediatric patients as young as 1 year of age with invasive aspergillosis and invasive mucormycotic.
FDA approval of bluebird bio’s Lyfgenia and Vertex Pharmaceuticals' and CRISPR Therapeutics’ Casgevy marks significant milestone in the treatment of sickle cell disease.
PREPVACC study expected to stop further HIV vaccination attempts after previously enrolling 1,500 participants in East and Southern Africa.
Migraine with comorbid obesity have been found to cause high levels of disability and are both more common in female patients.
Trial of Keytruda (pembrolizumab) plus chemotherapy and maintenance Lynparza (olaparib; AstraZeneca, MSD) for the treatment of metastatic squamous non-small cell lung cancer stopped after data did not show a survival benefit.

Fabhalta (iptacopan) is the first oral monotherapy approved by the FDA for the treatment of adult patients with paroxysmal nocturnal hemoglobinuria.

TAR-200 has a novel targeted releasing system for the treatment of patients with Bacillus Calmette-Guérin-unresponsive high-risk non-muscle-invasive bladder cancer who are ineligible for bladder removal surgery.
The FDA assigned a PDUFA date of April 5, 2024 for Opdivo (nivolumab) plus cisplatin-based chemotherapy for the first-line treatment of adults with unresectable or metastatic urothelial carcinoma.
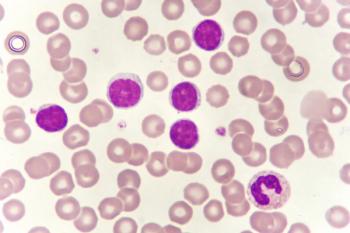
Jaypirca (pirtobrutinib) granted accelerated approval by the FDA for patients with chronic lymphocytic leukemia or small lymphocytic lymphoma who were previously administered least two prior lines of therapy that included a BTK inhibitor and a BCL2 inhibitor.
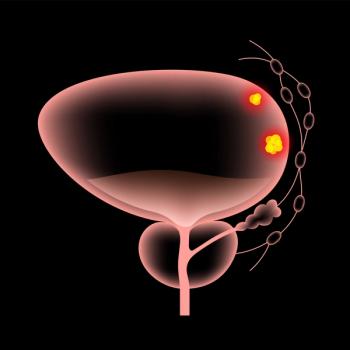
The FDA previously granted accelerated approval to the Keytruda plus Padcev combination for the treatment of patients with locally advanced or metastatic urothelial carcinoma who are ineligible for cisplatin-containing chemotherapy.
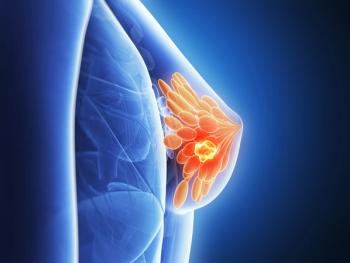
FDA to expedite review of zotatifin plus Faslodex (fulvestrant) and Verzenio (abemaciclib) as a second- or third-line treatment for patients with estrogen receptor–positive, human epidermal growth factor receptor 2-negative advanced or metastatic breast cancer whose disease progressed after treatment with endocrine therapy and a CDK4/6 inhibitor.
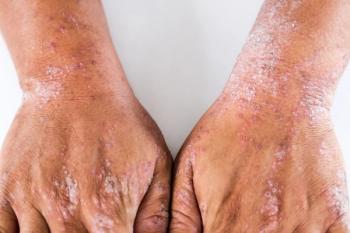
Supplemental new drug application for roflumilast cream 0.15% to treat atopic dermatitis in patients 6 years of age and older was assigned a Prescription Drug User Fee Act target action date of July 07, 2024.

KarXT (xanomeline-trospium) is currently in development to treat schizophrenia and psychosis related to Alzheimer disease.

Vivos becomes the first company to bring to market an alternative to continuous positive airway pressure (CPAP) or surgical neurostimulation implants for patients with severe OSA.
The launch of the Elecsys HBeAg quant immunoassay adds to Roche's viral hepatitis testing portfolio.

Ogsiveo is the first and only drug approved by the FDA to treat desmoid tumors.
Trial results expected to accelerate the potential of Dupixent to become the first FDA-approved treatment for chronic obstructive pulmonary disease.