
Regulatory
Latest News


The Center for Biologics Evaluation and Research is gearing up to facilitate the development and approval of regenerative medicine advanced therapies (RMAT), as defined by the 21st Century Cures Act.

The Senate confirms Scott Gottlieb as FDA commissioner. User fee reauthorizations, a hiring freeze, and the opioid epidemic are a few of the issues awaiting the agency's new leader.

There will be 13 full-time positions dedicated to creating and providing guidance in the emerging subject area.


The agency hopes to better use global resources and avoid duplicate inspections of foreign facilities.

Effectively addressing prior authorization challenges as part of a company’s core sales and marketing tactics can yield tangible and substantial benefits, writes Dan Rubin.



If pharma companies want to hold their own in the current climate, they must embrace rather than resist market change, writes Peter Muller.

Nine months on from the 2016 EFPIA Disclosure Code deadline - requiring all member companies across Europe to publish their data concerning their transfer-of-value transactions to HCPs - EFPIA's Andrew Powrie-Smith offered an update on media and industry responses.

Curing cancer is not an impossibility anymore. Essential to any effort to achieve this will be the policies coming from President Trump’s administration, writes James Nathanielsz.

Experts debate the practice’s rise up the life sciences agenda.

Amidst hundreds of presentations on new developments in cancer research and treatment discovery at the AACR meeting this week, FDA policies for speeding promising new therapies to patients garnered a good deal of attention.

Scott Gottlieb answers Senators' questions at his confirmation hearing before the Senate Health, Education, Labor and Pensions Committee.

Greater patient involvement in regulatory processes is raising questions about whether the views expressed by such groups fully reflect broader public needs and values.

Jill Wechsler outlines how the new FDA commissioner tasked with curbing regulation, speeding approvals-and collecting more fees to do it.

The development of highly effective cures for hepatitis C has prompted a panel of experts to propose an innovative financing arrangement to make these therapies more available to “neglected” populations in the U.S.

Despite the investment in place, industry still struggles to take advantage of competitive intelligence as key decision aid.

FDA efforts to speed more new generic drugs to market probably won’t do much to reduce high drug prices, writes Jill Wechsler.
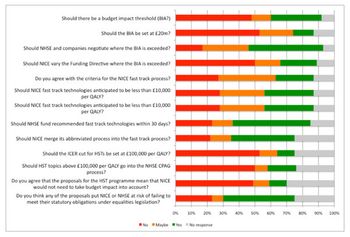
NICE and NHSE have been grappling with the issue of affordability. It’s not a new challenge, but with the advent of new treatments that are both cost effective and unaffordable, something has to be done. Leela Barham reports.

March 15, 2017

Trump’s choice for FDA commissioner faces drug pricing, regulatory, and approval challenges.

Rare Disease Day is a good moment to sound a warning that providers of rare medicines may be the next to land in the hot seat, writes Meg Alexander.

An FDA Memo addressing First Amendment legal issues regarding off-label use of medical products falls short of the necessary clarity, write Jamie Kendall and Alexandra Schulz.