
The Colorectal Cancer (CRC) therapeutics market in Asia-Pacific (APAC) is set to increase in value, from $1.9 billion in 2013 to $2.9 billion by 2020 (a Compound Annual Growth Rate [CAGR] of 6.5%), says a new report from GBI Research.

The Colorectal Cancer (CRC) therapeutics market in Asia-Pacific (APAC) is set to increase in value, from $1.9 billion in 2013 to $2.9 billion by 2020 (a Compound Annual Growth Rate [CAGR] of 6.5%), says a new report from GBI Research.

Two of the industry's most influential conferences occurred within weeks of each other this autumn – TEDMED and Health 2.0. Bill Drummy reviews both events.

Data analytic strategies can help companies capitalise on personalised medicine, writes Jill E. Sackman and Michael Kuchenreuther.

Will the FDA's social media guidelines help your digital strategy to take off? Check out what they say - and what they mean- in the latest issue of Pharm Exec Global Digest

Three medical affairs experts - Sean Turbeville, David A. Wells, and Charles Wolfus - review the capabilities of Google’s latest technology for delivering information to healthcare professionals in the field.

The med-tech industry has a legacy of creating life-extending products while rewarding investors. This connection between innovation and profitability has been a key driver. But, writes Doug Mowen, change is underway.

Bill Drummy reports on the key healthcare takeaways from this year's South by Southwest (SXSW) Interactive conference in Austin, Texas.

For pharma marketers, the good news is recent advances in social media monitoring make it possible to listen to or engage with patients on social media websites.

Recent research says social media and messaging apps are continuing to proliferate. But not so with senior execs, who are starting to avoid these networks altogether, says Chris Molloy.

Scignal Plus Taking the Noise Out of Signal Detection

Three years into the Apple-led tablet era, the iPad is still being used as no more than a glorified touchscreen. Gabriel Cangiano, Ron Kane, and David Windhausen look at the key role it can play in the future of pharma.

How to Implement an ePRO Strategy

Mukhtaer Ahmed outlines how advances in machine-to-machine (M2M) technologies have the potential to transform clinical development programs, including clinical trials.

Are electronic health records a viable channel for engagement? Ben Comer reports.

How vulnerable is Big Pharma to the predations of organized terrorist groups or that rogue malcontent with an agenda to wreak havoc on society?

Pharmacovigilance in a box
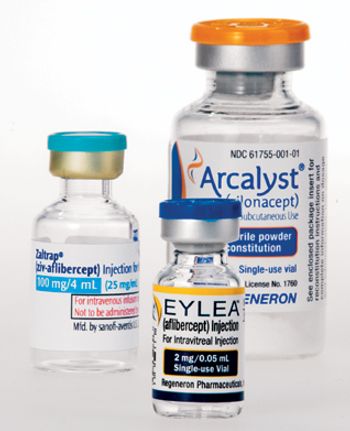
Led by two New Yorkers with a mindset that marries novel scientific insights to the processing power of novel technologies, Regeneron's success is a revolutionary challenge to the industry status quo.

For the hard-pressed oncology practice, new technologies hold the key to surviving-and thriving.

Improving the quality of clinical trials is a major strategic activity for Big Pharma, one where efficient management of a demanding set of regulatory requirements can have a positive impact on the bottom line.
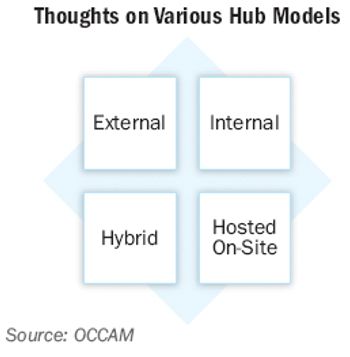
Innovation in hub program design for patient scrip data and clinical support services can lead to increased market share in the hotly contested specialty medicine space.

Ben Comer profiles 10 innovative technologies designated as new and revolutionary tools for the treatment of disease and disability.

If we swallow the hype and decide that we all need to get onto the mobile app bandwagon, how exactly do we go about it? Sven Awege explains.
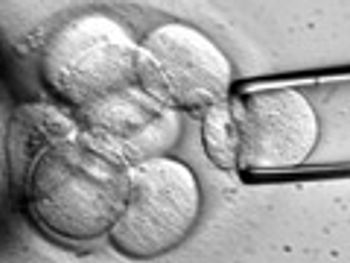
Ideology, politics, and a stilted political debate may be causing pharma to overlook the potential of emerging stem cell therapies in fostering a new generation of cures.

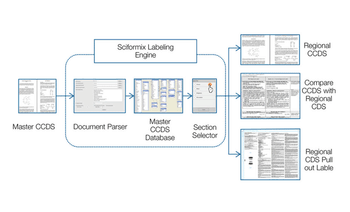
Sciformix's Integrated Drug Safety solution seamlessly interweaves and synchronizes services across all phases of the product lifecycle.