
Since its launch two years ago, the iPad has enjoyed unprecedented success in the healthcare space. But how long can the technology captivate physicians? Dan Goldsmith and Paul Shawah report.

Since its launch two years ago, the iPad has enjoyed unprecedented success in the healthcare space. But how long can the technology captivate physicians? Dan Goldsmith and Paul Shawah report.

The EDQM are looking to recruit a Scientific Programme Officer to join the team in co-ordinating and running mutual joint visits and audits of laboratories within the Official Medicines Control Laboratories (OMCLs) for a five year duration.
Early efforts in the pharma space to harness the engagement factor in game technology focused on delivering a fun experience, with a tackedon educational component. Today's games bring behavior change to the forefront, incorporating social dynamics, mobility, and increasingly sophisticated mechanics.

Options for using data have proliferated to the point of becoming overwhelming. So what opportunities does this information explosion present?

Highlighting the companies awarded for exemplifying a high level of operational excellence with technology.

Defining and using quality metrics for adverse events case processing.

This year's Ad Stars embrace creativity through the eyes of the patients. Ben Comer and Jennifer Ringler report.

While what is considered 'traditional' marketing has evolved over the years, it's clear that a digital strategy is an absolute necessity to compete today-not just tomorrow.

NNIT's GxP Cloud Now Offers SaaS

Jim Currie and Baber Ghauri talk to Pharm Exec about making connections that resonate with audiences in a digital world.

Making real connections that resonate with audiences in a digital world is a fine art, especially in the heavily regulated world of Big Pharma

So far companies have only scratched the surface of the potential offered by the 'new mobile ecosystem', writes Neeraj Singhal.
For a rendering to be effective, the right balance must be struck between captivating art and accurate science.
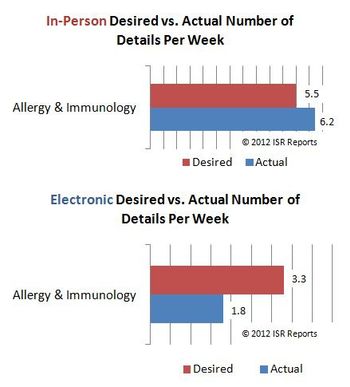
Forget illegal immigrants, it is machines that are stealing American jobs. For some medical practice areas, however, digital details haven’t sufficiently stepped in where their human sales rep predecessors have stepped (or have been pushed) out, according to a survey of U.S. physicians.
A selection of the recent breakthrough technologies showcased at the Cleveland Clinic's Medical Innovations Summit.

The time is right for industry to overcome its social media phobia once and for all.

Richard Barker, former Director General of ABPI, proposes a new agenda on how to restore public confidence in the value behind science.
Social media now gives companies a plethora of creative ways to digitally spread recent company news and keep customers informed
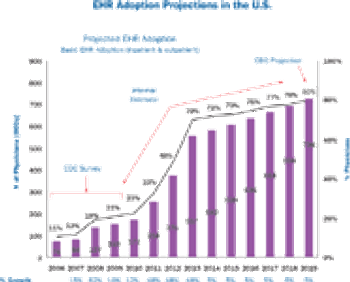
It's a work in progres among EHR providers to offer a clear bridge between pharma and physicians.

Is Google+ the social media platform that pharma has been waiting for?

'Like' and 'Dislike' practices in the virtual Facebook pharmaceutical world.

NeHC's new Consumer Consortium is promoting a new stakeholder agenda to improve patient knowledge

A federal appeals court has lifted a ban on federal funding for embryonic stem cell research.

Online sentiment analysis monitoring can help biopharma resource managers make crucial decisions

The sleek, portable iPad, coupled with customized apps, gives sales reps a leg up